Dr Masood Entezari Asl ANESTHESIA FOR PEDIATRICS INTRODUCTION


















































- Slides: 89


Dr Masood Entezari. Asl ANESTHESIA FOR PEDIATRICS

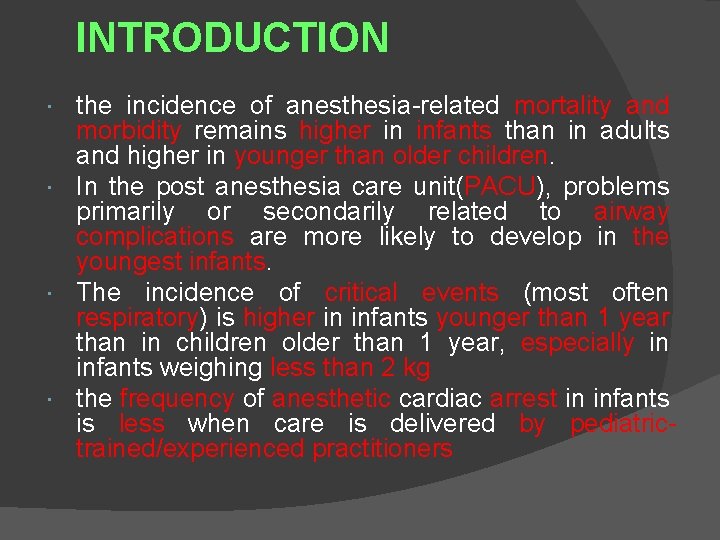
INTRODUCTION the incidence of anesthesia-related mortality and morbidity remains higher in infants than in adults and higher in younger than older children. In the post anesthesia care unit(PACU), problems primarily or secondarily related to airway complications are more likely to develop in the youngest infants. The incidence of critical events (most often respiratory) is higher in infants younger than 1 year than in children older than 1 year, especially in infants weighing less than 2 kg the frequency of anesthetic cardiac arrest in infants is less when care is delivered by pediatrictrained/experienced practitioners

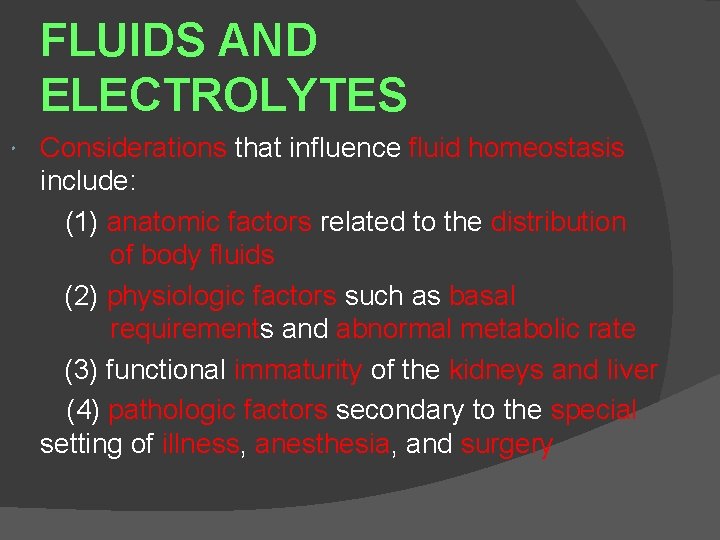
FLUIDS AND ELECTROLYTES Considerations that influence fluid homeostasis include: (1) anatomic factors related to the distribution of body fluids (2) physiologic factors such as basal requirements and abnormal metabolic rate (3) functional immaturity of the kidneys and liver (4) pathologic factors secondary to the special setting of illness, anesthesia, and surgery

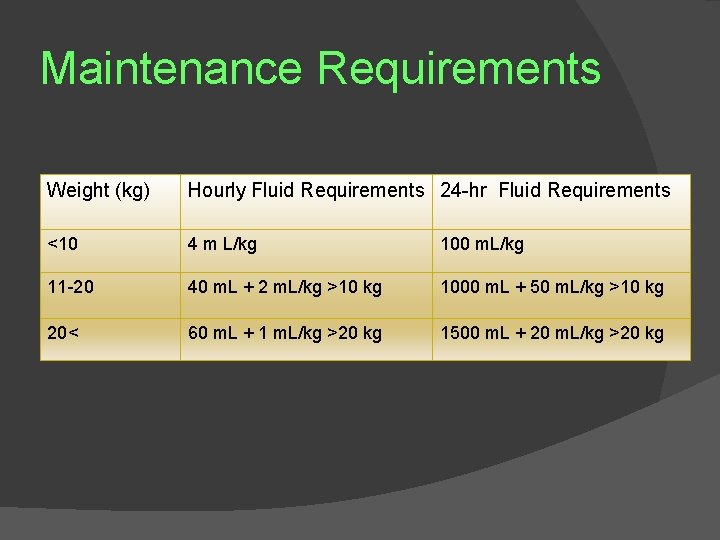
Maintenance Requirements Weight (kg) Hourly Fluid Requirements 24 -hr Fluid Requirements <10 4 m L/kg 100 m. L/kg 11 -20 40 m. L + 2 m. L/kg >10 kg 1000 m. L + 50 m. L/kg >10 kg 20< 60 m. L + 1 m. L/kg >20 kg 1500 m. L + 20 m. L/kg >20 kg

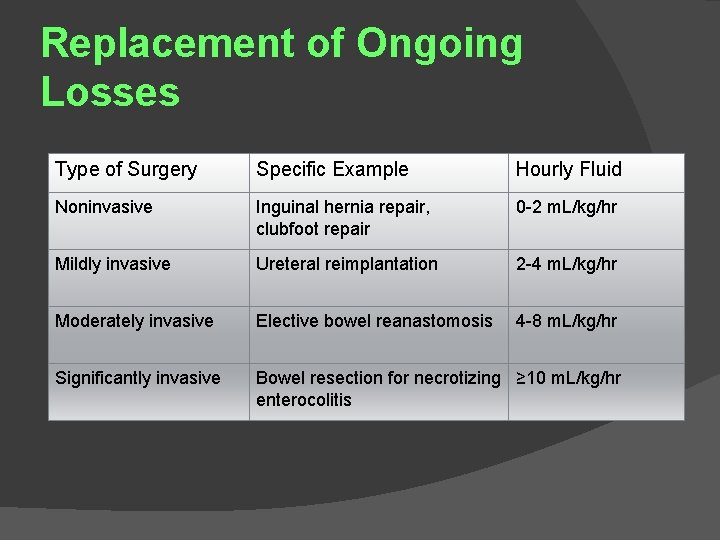
Replacement of Ongoing Losses Type of Surgery Specific Example Hourly Fluid Noninvasive Inguinal hernia repair, clubfoot repair 0 -2 m. L/kg/hr Mildly invasive Ureteral reimplantation 2 -4 m. L/kg/hr Moderately invasive Elective bowel reanastomosis 4 -8 m. L/kg/hr Significantly invasive Bowel resection for necrotizing ≥ 10 m. L/kg/hr enterocolitis

Clinically Important Formulas for Perioperative Management of Children Volume of Packed Red Blood Cells (PRBCs) PRBCs(m. L) = EBV x Desired. Hctx (Actual Hct/Hct of PRBCs) EBV, estimated blood volume; Hct, hematocrit Intraoperative Fluid Administration (Consider Four Factors) 1. "Catch up" (maintenance rate x hours NPO) 2. Maintenance fluid 3. Ongoing losses (blood loss, third spacing) 4. Special considerations (calcium, glucose, coagulation factors) Intraoperative Glucose Maintenance requirement of glucose for newborn (4 mg/kg/min = 240 mg/kg/hr) Maintenance fluid (Ds = 50 mg glucose/m. L)(4 m. L/kg/min = 200 ml/kg/hr) Delivery of a Dissolution at a rate greater than 4 m. L/kg/hr may lead to hyperglycemia; preoperative evaluation includes noting the blood glucose concentration (Chemstrip/Dextrostix) level at a specific infusion (mg/kg/min) of glucose

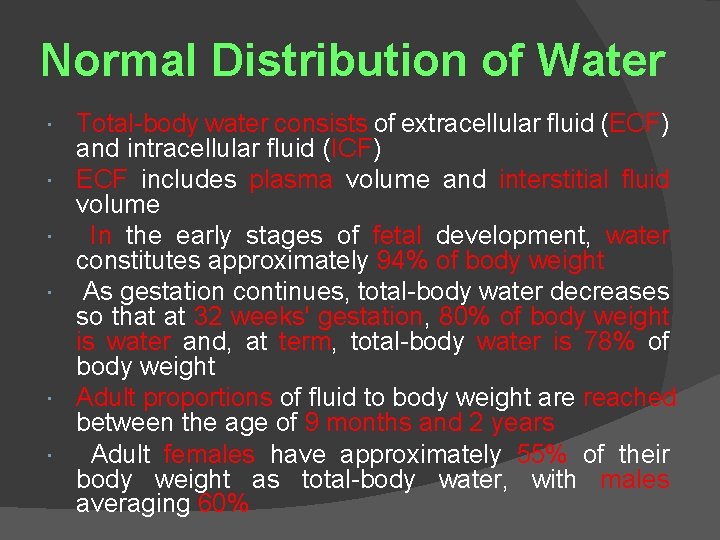
Normal Distribution of Water Total-body water consists of extracellular fluid (ECF) and intracellular fluid (ICF) ECF includes plasma volume and interstitial fluid volume In the early stages of fetal development, water constitutes approximately 94% of body weight As gestation continues, total-body water decreases so that at 32 weeks' gestation, 80% of body weight is water and, at term, total-body water is 78% of body weight Adult proportions of fluid to body weight are reached between the age of 9 months and 2 years Adult females have approximately 55% of their body weight as total-body water, with males averaging 60%


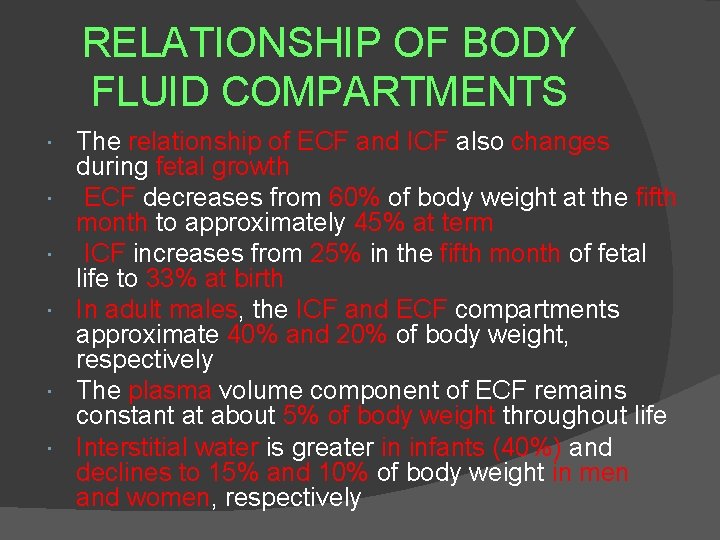
RELATIONSHIP OF BODY FLUID COMPARTMENTS The relationship of ECF and ICF also changes during fetal growth ECF decreases from 60% of body weight at the fifth month to approximately 45% at term ICF increases from 25% in the fifth month of fetal life to 33% at birth In adult males, the ICF and ECF compartments approximate 40% and 20% of body weight, respectively The plasma volume component of ECF remains constant at about 5% of body weight throughout life Interstitial water is greater in infants (40%) and declines to 15% and 10% of body weight in men and women, respectively

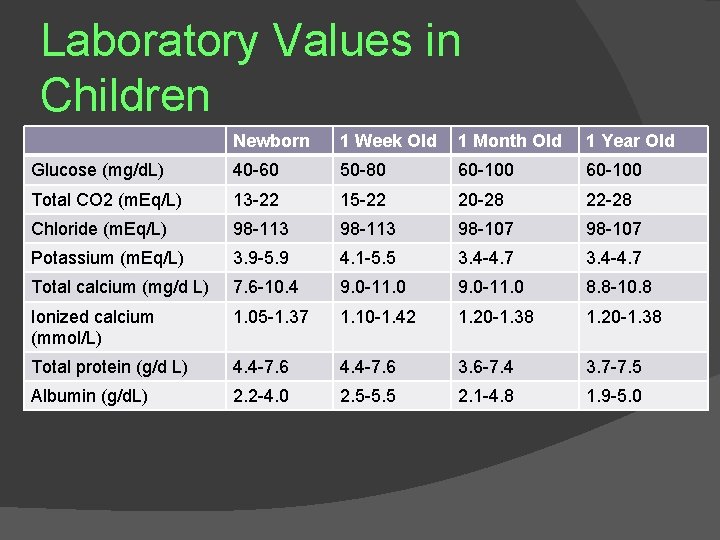
ELECTROLYTECOMPOSITIO N OF BODY FLUIDS The electrolyte composition of the body fluids of infants is also different from that in adults Higher plasma chloride and lower bicarbonate and p. H imply a mild metabolic acidosis with reduced buffering power (Tables) In the first 10 days of postnatal life (term infants), serum potassium levels may be as high as 6. 0 to 6. 5 m. Eql. L In term infants, serum potassium ranges from 3. 5 to 5. 5 m. Eql. L after the first 2 to 3 weeks of life Another unique feature of newborns is the reduced protein concentration, which results in lower intravascular oncotic pressure

Laboratory Values in Children Newborn 1 Week Old 1 Month Old 1 Year Old Glucose (mg/d. L) 40 -60 50 -80 60 -100 Total CO 2 (m. Eq/L) 13 -22 15 -22 20 -28 22 -28 Chloride (m. Eq/L) 98 -113 98 -107 Potassium (m. Eq/L) 3. 9 -5. 9 4. 1 -5. 5 3. 4 -4. 7 Total calcium (mg/d L) 7. 6 -10. 4 9. 0 -11. 0 8. 8 -10. 8 Ionized calcium (mmol/L) 1. 05 -1. 37 1. 10 -1. 42 1. 20 -1. 38 Total protein (g/d L) 4. 4 -7. 6 3. 6 -7. 4 3. 7 -7. 5 Albumin (g/d. L) 2. 2 -4. 0 2. 5 -5. 5 2. 1 -4. 8 1. 9 -5. 0

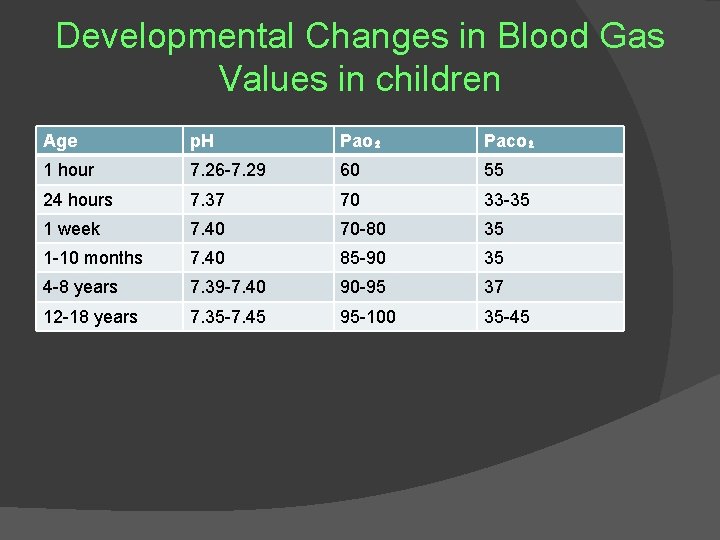
Developmental Changes in Blood Gas Values in children Age p. H Pao₂ Paco₂ 1 hour 7. 26 -7. 29 60 55 24 hours 7. 37 70 33 -35 1 week 7. 40 70 -80 35 1 -10 months 7. 40 85 -90 35 4 -8 years 7. 39 -7. 40 90 -95 37 12 -18 years 7. 35 -7. 45 95 -100 35 -45

Over the first week of life, water exchange is often negative because of ongoing losses through the skin, lungs, and urine in the setting of limited intake A 7 -kg infant with a 21 OO-m. L ECF volume takes in and excretes approximately 700 to 1000 m. L of fluid daily, which represents a 33% turnover in ECF By comparison, a 70 -kg adult with a 14, 000 -m. L ECF volume excretes approximately 2000 m. L of fluid daily for a 14% turnover This high rate of fluid exchange may expose infants to more rapid development of both dehydration and over hydration

PEDIATRIC AIRWAY Preoperatively, the anesthesiologist must meticulously assess all aspects of the airway to develop detailed and flexible plans for (1) intubating the trachea, (2) intraoperative airway management (3) postoperative recovery (Table) The anesthetic plan for management of the child's airway may be influenced by the site of surgery Maintaining upper airway patency is an active process that is depressed during general anesthesia During spontaneous ventilation, the upper airway is exposed to potentially collapsing negative pressure during inspiration The pharynx is prone to collapse because negative pressure pulls the tongue against the pharynx General anesthesia depresses activity of the upper airway and thereby predisposes to oropharyngeal obstruction

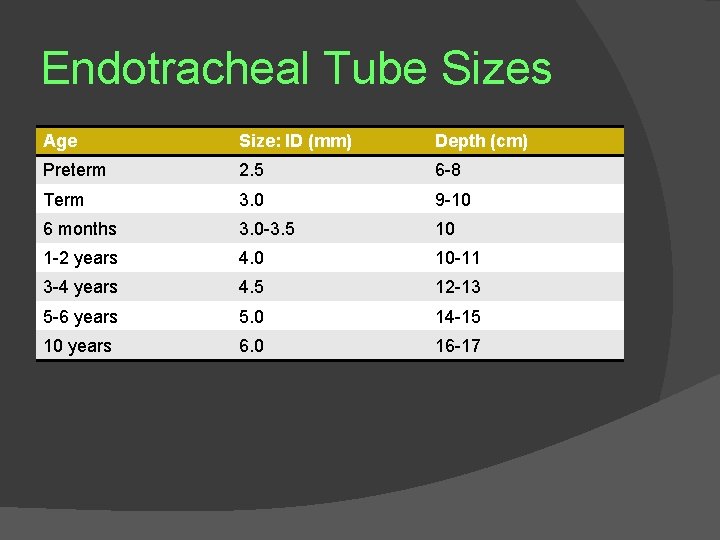
Endotracheal Tube Sizes Age Size: ID (mm) Depth (cm) Preterm 2. 5 6 -8 Term 3. 0 9 -10 6 months 3. 0 -3. 5 10 1 -2 years 4. 0 10 -11 3 -4 years 4. 5 12 -13 5 -6 years 5. 0 14 -15 10 years 6. 0 16 -17

The contribution of the tongue to airway obstruction is exaggerated in infants because the tongue is large relative to the total volume of the mouth Infants or children with small upper airways secondary to craniofacial anomalies, weakness as a result of neuromuscular or central nervous system disorders, impingement on the airway secondary to tumors or hemangiomas, or dysfunction of the tracheobronchial tree because of an upper respiratory infection (URI) are especially prone to pharyngeal obstruction by the tongue

Head position is important in maintaining upper airway patency during anesthesia Flexing an infant's head may cause the upper airway to collapse more readily In addition, the small, soft airways of neonates (especially premature neonates) are more compressible if the neck is flexed Extending or keeping the neck in a neutral position while applying positive airway pressure during ventilation with a bag and facemask is important, particularly during induction of general anesthesia

Unique Anatomic Features The larynx of infants is higher in the neck (C 3 -4) than in adults (C 4 -5) An infant's epiglottis is large, but it is narrow and short A straight laryngoscope blade may allow the larynx of a normal infant to be visualized more easily When laryngeal anatomy is distorted by craniofacial anomalies (micrognathia or midface hypoplasia), direct visualization of the larynx may be impossible, and alternative methods of securing the airway should be available An infant's vocal cords are slanted such that the posterior commissure is more cephalad than the anterior commissure

This arrangement may predispose the anterior sublaryngeal airway to trauma from an endotracheal tube The subglottic area is prone to traumatic injury from an endotracheal tube because the narrowest portion of an infant's larynx is at the cricoid cartilage In adults, the narrowest portion is the glottic rim Thus, an endotracheal tube that easily passes through the vocal cords of an infant or child may fit snugly in the subglottis and cause subglottic edema and symptoms of increased airway resistance after tracheal extubation This increased resistance is usually reversible, but subglottic stenosis may develop after prolonged tracheal intubation with an oversized endotracheal tube

Airway Assessment Difficult tracheal intubation generally occurs when facial or oral pathology prevents visualization of the larynx or when the larynx is easily visualized by direct laryngoscopy but a lesion in the supraglottic, or subglottic region interferes with insertion of the endotracheal tube When the past medical history documents previous difficult airway management and tracheal intubation, it is recommended that a physician experienced in performing pediatric bronchoscopy be present during initial airway management Fiberoptic airway endoscopy with or without the aid of a laryngeal mask airway (LMA) may be indicated for securing a difficult airway In some circumstances, it may be prudent to have available a surgeon skilled in performing cricothyrotomy or tracheostomy (or both) In some situations, performing a controlled tracheostomy may be less traumatic than persisting with multiple attempts at direct laryngoscopy

DEVELOPMENTAL PHYSIOLOGY

DEVELOPMENTAL PHYSIOLOGY Respiratory System Circulatory System Renal Function Hematology

Respiratory System (Table) Lung Development Alveoli develop mainly after birth and increase from 20 million terminal air sacs in a newborn to approximately 300 million alveoli at 18 months of age In general, extra uterine viability is first likely after 26 weeks when the respiratory saccules have developed and vascularization by capillaries has occurred Supportive care of a premature infant commonly includes oxygen and positive pressure ventilation, and infections are inevitable

Comparison of Pulmonary Variables Neonate Infant 5 Years of Age Adult Weight (kg) 3 4 -10 18 70 Breathing frequency (breaths/min) 35 24 -30 20 15 Tidal volume (m. L/kg) 6 6 Vital capacity (m. L/kg) 35 70 Alveolar ventilation (m. L/kg/min) 130 60 Carbon dioxide production (ml/kg/min) 6 3 Functional residual capacity (m. L/kg) 25 25 35 40

RIB CAGE The compliant rib cage of a newborn produces a mechanical disadvantage to effective ventilation The negative intrapleural pressure produced by normal inspiratory effort tends to collapse the cartilaginous, compliant chest of an infant (especially a premature newborn), which causes paradoxical chest wall motion and limits airflow during inspiration The circular configuration of the rib cage (ellipsoid in adults) and the horizontal angle of insertion of the diaphragm (oblique in adults) cause distortion of a newborn's rib cage and inefficient diaphragmatic contraction.

DIAPHRAGM An adult diaphragm contains 55% type I fibers (fatigue resistant, slow-twitching, highly oxidative fibers), whereas the diaphragm of a full-term infant has 25% and that of a preterm infant has 10% A lower proportion of type I fibers predisposes these primary respiratory muscles to fatigue The intercostal muscles show a similar developmental pattern

PULMONARY SURFACTANT Pulmonary surfactant effects dramatic changes in lung mechanics, including distensibility and endexpiratory volume stability The development of respiratory distress syndrome of the newborn correlates with insufficient (premature infants) or delayed (infants of diabetic mothers) synthesis of surfactant The most significant decrease in infant mortality observed in 20 years in the United States occurred in 1990, the year that surfactant was released commercially However, chronic lung disease persists as a common problem in approximately 20% of premature infants as a result of the complex interplay of many factors in addition to surfactant during normal growth and development of the lungs

Circulatory System

FETAL CIRCULATION The fetal circulation is characterized by: (1) increased pulmonary vascular resistance (2) decreased pulmonary blood flow (3) decreased systemic vascular resistance (4) right-to-left blood flow through the patent ductus arteriosus and foramen ovale At birth, the onset of spontaneous ventilation and elimination of the placental circulation decrease pulmonary vascular resistance and increase pulmonary blood flow Simultaneously, systemic vascular resistance increases, left atrial pressure increases, the foramen ovale closes functionally, and the right-to left shunting ceases When anatomic closure is achieved and cardiac anatomy is normal shunting through the ductus arteriosus is eliminated

Arterial hypoxemia or acidosis in a newborn can precipitate return to a fetal pattern of circulation (pulmonary arterial vasoconstriction, pulmonary hypertension, reduced pulmonary blood flow) This combination leads to right atrial pressure increasing above left atrial pressure and thereby results in right-to-left shunting through the foramen ovale and ductus arteriosus This return to a fetal circulatory pattern, termed persistent fetal circulation or persistent pulmonary hypertension of the newborn, further exacerbates the arterial hypoxemia and acidosis

MYOCARDIAL FUNCTION The relative noncompliance of the neonatal heart implies a limited capacity to handle a volume load or to increase stroke volume for augmentation of cardiac output (or both) Thus, the "Frank-Starling" responses considered to play a limited role, whereas the heart rate is critical for maintaining cardiac output in a newborn Over the first months of life, myocardial contractility gradually increases, which allows cardiac output to be maintained over a wide range of preload and after load

EVALUATIONOF CARDIOPULMONARY initial step. FUNCTION in evaluating the cardiopulmonary The system of a newborn begins with the physical examination Skin color, capillary filling time, trends in blood pressure, heart rate, intensity of peripheral pulses, presence of a murmur or S 3 or S 4 heart sounds, respiratory rate, effort, and breath sounds, as well as decreased urine output or metabolic acidosis, should be assessed Interpretation of the electrocardiogram, chest radiograph, and echocardiogram will allow rational planning for intraoperative monitoring, selection of anesthetic drugs, delivery of intravenous fluids, postoperative recovery, and the extent of the proposed surgical procedure (total correction or staged procedure)

Comparison of Cardiovascular Variables Neonate Infant 5 Years of Adult Age Weight 3 4 -10 18 70 Oxygen consumption (mg/kg/min) 6 5 4 3 Systolic blood pressure (mm Hg) 65 90 -95 95 120 Heart rate (beats/min) 130 120 90 80

Renal Function

Renal Function Urine production increases from about 5 ml/hr at 20 weeks, to about 18 ml/hr at 30 weeks, to about 50 ml/hr at 40 weeks of gestation Although the kidneys are not essential for maintaining normal fluid and electrolyte balance in a fetus, urine production contributes to normal amniotic fluid volume and is critical for normal pulmonary and urinary tract development

GLOMERULAR FILTRATION RATE The renal function of a newborn versus an adult is characterized by a decreased glomerular filtration rate (GFR), decreased excretion of solid materials, and decreased urine concentrating ability The GFR increases with gestational age, and by 34 to 36 weeks of gestation, values are similar to those reported for full-term infants Over the first 3 months of life, the GFR increases twofold to threefold A slower rise is noted until adult values are reached by 12 to 24 months of life

RENAL TUBULAR FUNCTION Limited renal tubular reabsorptive function is the basis for the loss of bicarbonate and the "normal" acidosis that occur in a newborn, particularly premature newborns (sometimes called renal tubular acidosis type 4) Similarly, proximal renal tubular reabsorption of sodium increases with gestational age Of note, arterial hypoxemia, respiratory distress, and hyper bilirubinemia can increase fractional sodium excretion The limited distal renal tubular function also impairs the ability of the kidneys to excrete a sodium load. In addition, tubular immaturity affects the conservation of amino acids, nucleosides, glucose, and other essential substrates

Hematology

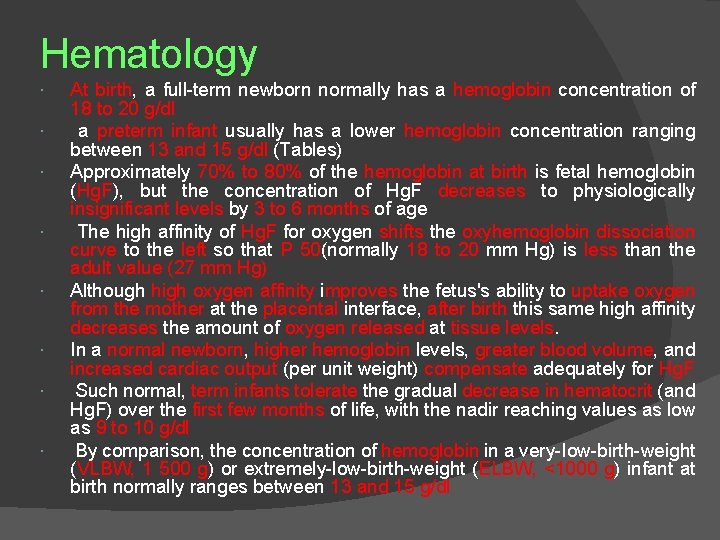
Hematology At birth, a full-term newborn normally has a hemoglobin concentration of 18 to 20 g/dl a preterm infant usually has a lower hemoglobin concentration ranging between 13 and 15 g/dl (Tables) Approximately 70% to 80% of the hemoglobin at birth is fetal hemoglobin (Hg. F), but the concentration of Hg. F decreases to physiologically insignificant levels by 3 to 6 months of age The high affinity of Hg. F for oxygen shifts the oxyhemoglobin dissociation curve to the left so that P 50(normally 18 to 20 mm Hg) is less than the adult value (27 mm Hg) Although high oxygen affinity improves the fetus's ability to uptake oxygen from the mother at the placental interface, after birth this same high affinity decreases the amount of oxygen released at tissue levels. In a normal newborn, higher hemoglobin levels, greater blood volume, and increased cardiac output (per unit weight) compensate adequately for Hg. F Such normal, term infants tolerate the gradual decrease in hematocrit (and Hg. F) over the first few months of life, with the nadir reaching values as low as 9 to 10 g/dl By comparison, the concentration of hemoglobin in a very-Iow-birth-weight (VLBW, 1 500 g) or extremely-low-birth-weight (ELBW, <1000 g) infant at birth normally ranges between 13 and 15 g/dl

Of note, the nadir of a premature (<30 weeks' gestation) infant's hemoglobin may be as low as 6 to 7 g/dl by 3 to 4 months of age Erythropoietin is now routinely administered to infants in the neonatal intensive care unit, thereby avoiding such profound anemia Newborns with cardiovascular or respiratory instability often benefit from a hematocrit higher than 40% to 45% to facilitate adequate oxygen delivery Blood loss exceeding 10% to 15% of blood volume (or even less in some patients) may not be tolerated by newborns, especially VLBW infants Cross-matched blood should be available for surgery in a newborn, especially when blood loss is anticipated Assessment of clotting function should be considered before major surgery in a newborn because the synthesis of prothrombin and factors II, VII, and X is limited in an immature liver Perinatal asphyxia and septicemia affect the function and concentration of both clotting factors and platelet count and thereby result in coagulopathies Before surgical intervention, the availability of fresh frozen plasma, fibrinogen, and platelets must be considered

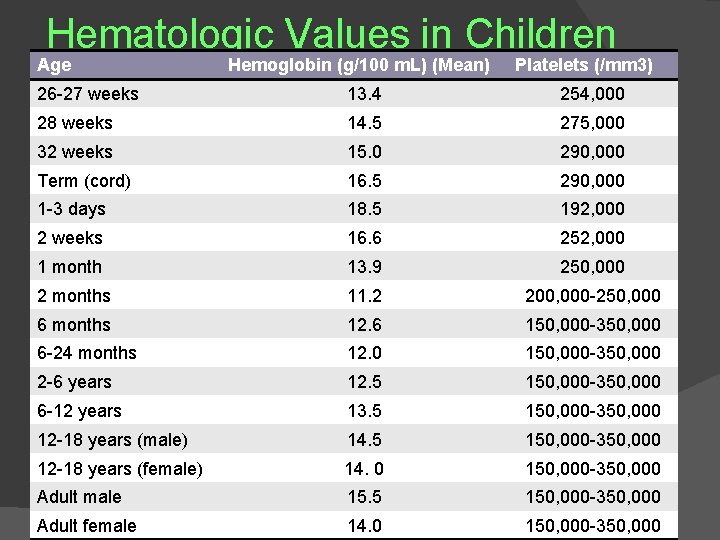
Hematologic Values in Children Age Hemoglobin (g/100 m. L) (Mean) Platelets (/mm 3) 26 -27 weeks 13. 4 254, 000 28 weeks 14. 5 275, 000 32 weeks 15. 0 290, 000 Term (cord) 16. 5 290, 000 1 -3 days 18. 5 192, 000 2 weeks 16. 6 252, 000 1 month 13. 9 250, 000 2 months 11. 2 200, 000 -250, 000 6 months 12. 6 150, 000 -350, 000 6 -24 months 12. 0 150, 000 -350, 000 2 -6 years 12. 5 150, 000 -350, 000 6 -12 years 13. 5 150, 000 -350, 000 12 -18 years (male) 14. 5 150, 000 -350, 000 12 -18 years (female) 14. 0 150, 000 -350, 000 Adult male 15. 5 150, 000 -350, 000 Adult female 14. 0 150, 000 -350, 000

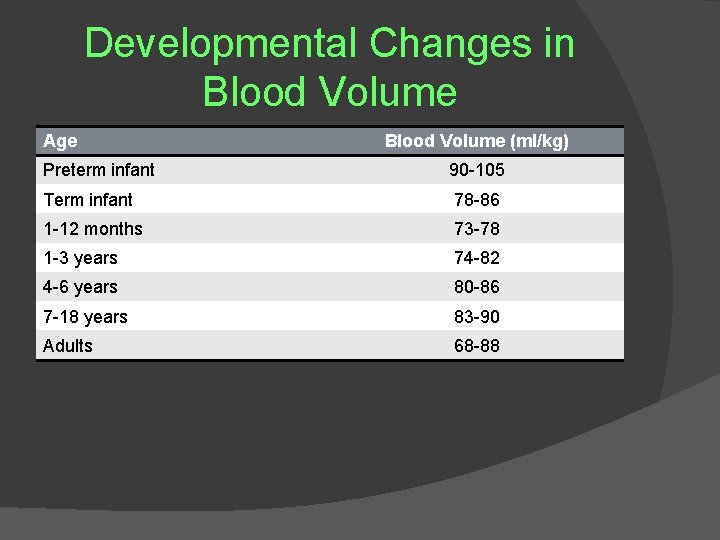
Developmental Changes in Blood Volume Age Blood Volume (ml/kg) Preterm infant 90 -105 Term infant 78 -86 1 -12 months 73 -78 1 -3 years 74 -82 4 -6 years 80 -86 7 -18 years 83 -90 Adults 68 -88

MEDICAL AND SURGICAL DISEASES THAT AFFECT THE NEWBORN

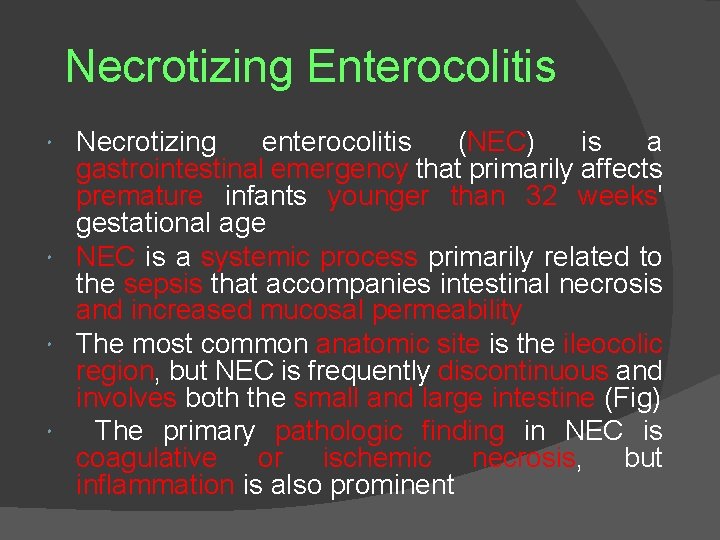
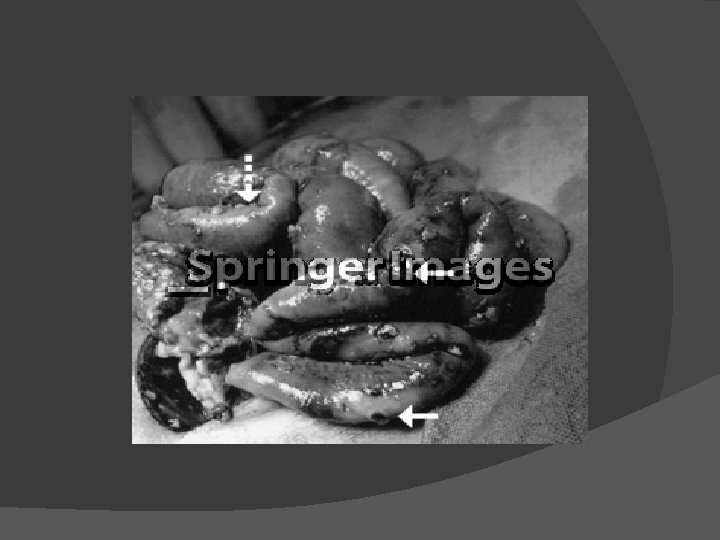
Necrotizing Enterocolitis Necrotizing enterocolitis (NEC) is a gastrointestinal emergency that primarily affects premature infants younger than 32 weeks' gestational age NEC is a systemic process primarily related to the sepsis that accompanies intestinal necrosis and increased mucosal permeability The most common anatomic site is the ileocolic region, but NEC is frequently discontinuous and involves both the small and large intestine (Fig) The primary pathologic finding in NEC is coagulative or ischemic necrosis, but inflammation is also prominent



CLINICAL MANIFESTATIONS Typically, a preterm baby in whom NEC develops has a history of perinatal asphyxia or postnatal cardiorespiratory instability and manifests gastrointestinal signs between the 1 st and 10 th days of life (abdominal distention, retained gastric secretions that may be bile tinged, vomiting, bloody or mucoid diarrhea, and occult blood loss in stools) Bowel necrosis and perforation may develop and be accompanied by sepsis with thermal instability, lethargy, metabolic acidosis, hypotension, hypoxia, jaundice, disseminated intravascular coagulation(DIC), and generalized bleeding Most infants with NEC have a decreased platelet count (50, 000 to 75, OOO/mm 3) and prolonged prothrombin(PT) and partial thromboplastin times(PTT) Abdominal radiographs may reveal dilated, fixed (adynamic ileus) loops of bowel, gas in the portal venous system, and pneumoperitoneum

TREATMENT Unless there is evidence of intestinal necrosis or perforation, the initial treatment of NEC is nonoperative and includes: - decompression of the stomach - cessation of feeding - broad-spectrum antibiotics - fluid and electrolyte therapy - parenteral nutrition - correction of hematologic abnormalities Inotropic drugs may be needed to maintain hemodynamic stability Bowel perforation is the most important indication for surgery

Other events that increase the likelihood for surgical intervention include: - peritonitis - air in the portal system - bowel wall edema - ascites -progressively deteriorating hemodynamic status

MANAGEMENT OF ANESTHESIA The preoperative assessment of infants with NEC should focus on evaluating and correcting the respiratory, circulatory, metabolic, and hematologic disorders During fluid resuscitation these infants must be monitored carefully for signs of patent ductus arteriosus or congestive heart failure Most require mechanical ventilatory support because of metabolic acidosis and respiratory complications of fluid administration and sepsis Depending on the hemodynamic status of the infant, intraoperative monitoring may include continuous monitoring of intra-arterial and central venous pressure and blood gas analysis At a minimum, intravenous access should be adequate to allow vigorous crystalloid and colloid administration Fresh frozen plasma(FFP), platelets, and red blood cells may be administered early in surgery in response to blood loss, hemodynamic instability, or coagulopathy In preterm infants, inspired oxygen concentrations should be adjusted to produce a Pao₂ of 50 to 70 mm Hg (Spo₂ of 90% to 95%) Nitrous oxide should be avoided, especially in the presence of free

Volatile anesthetics are often poorly tolerated and should be introduced only in low concentrations to supplement intravenous drugs (opioids, ketamine) Fentanyl or remifentanil combined with low doses of volatile anesthetics can provide analgesia and amnesia and allow cardiovascular stability Neuromuscular blocking drugs facilitate surgical exposure Inotropes are occasionally needed to support the cardiovascular system when fluid therapy alone fails to maintain adequate perfusion Observing bowel perfusion during surgery may provide a useful guide to the effectiveness of fluid and inotropic support Postoperatively, mechanical ventilation and cardiovascular support are usually required in the neonatal intensive care unit(NICU) Parenteral nutrition is essential after sepsis is controlled and metabolic stability is established

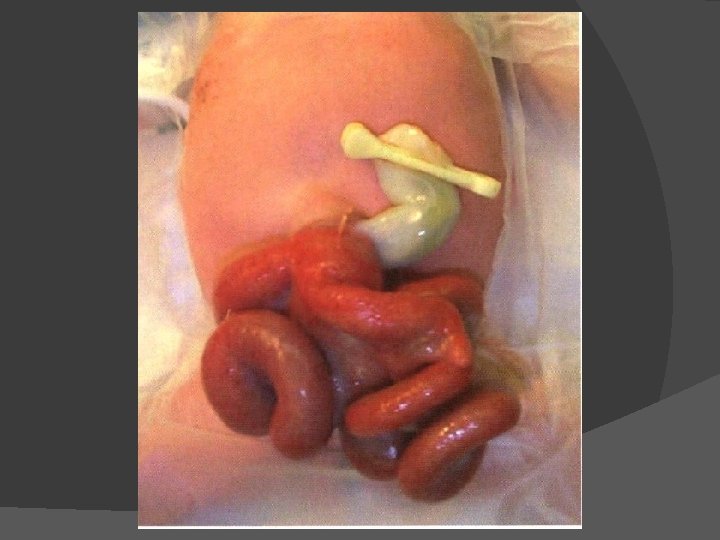
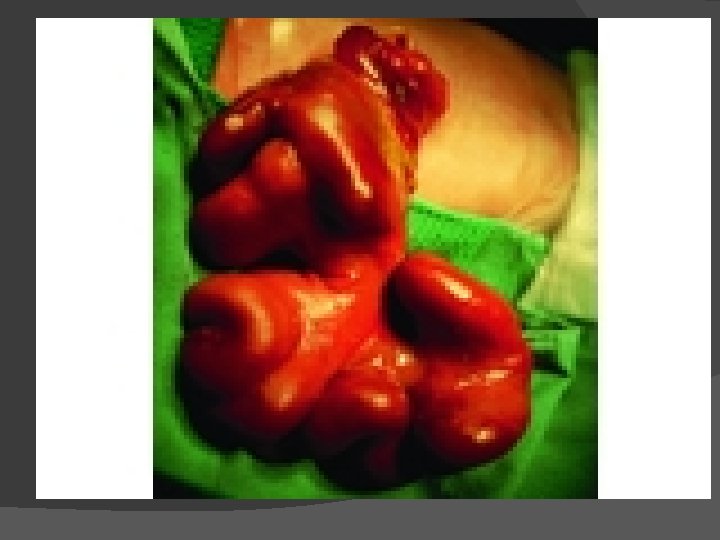
Abdominal Wall Defects Omphalocele and gastroschisis are distinct lesions despite a similar physical appearance (Figs) Omphalocele is a central defect of the umbilical ring, and the abdominal contents are within a sac, unless the sac ruptures in utero Gastroschisis is an abdominal wall defect, usually to the right of the umbilical cord This defect is generally larger than 5 cm in diameter and typically contains only large and small bowel (rarely the liver may exit through the defect) The bowel is exposed to the intrauterine environment with no sac, so the loops are matted, thickened, and often covered with an inflammatory coating or peel This anatomic defect can be diagnosed in utero with fetal ultrasound





TREATMENT Preoperative management of abdominal wall defects is directed primarily toward fluid resuscitation and minimizing heat loss, treating sepsis, avoiding direct trauma to the herniated organs, and identifying other anomalies A bowel bag may be used to minimize fluid and heat loss Decompression of the stomach with an orogastric or nasogastric tube is important to prevent regurgitation, aspiration pneumonia, and further bowel distention

Fluid Therapy Intravenous fluid therapy (as much as two to four times [150 to 300 ml/kg/day] the usual maintenance infusion rate [80 to 100 ml/kg/day]) is infused to ensure adequate hydration and to compensate for peritonitis, bowel ischemia, and significant third-space loss Initially, a balanced salt solution is used, and urine output is monitored Urine output of 1 to 2 ml/kg/hr suggests adequate hydration Because of the large fluid requirements, acidbase status and electrolyte levels should be monitored Rarely, colloid is required to maintain hemodynamic stability preoperatively

Surgical Treatment Surgical management is aimed at repairing the abdominal wall defect and reducing the protruded viscera Forcing the viscera into an underdeveloped abdominal cavity that cannot easily accommodate the herniated bowel can have dramatic effects on ventilation and oxygenation, as well as on cardiac output and systemic blood pressure (secondary to impaired venous return from the inferior vena cava) Excessive compression of the intestine can produce ischemia Thus, during abdominal closure, the anesthesiologist must anticipate the potential effects of decreased pulmonary compliance (increased airway pressure, decreased oxygen saturation, hypercapnia) and inadequate cardiac output If primary closure is not possible, a Silastic mesh prosthesis ("silo") is sutured to the fascia of the defect After the silo is in place, the extra-abdominal organs are then gradually returned to the peritoneal cavity over a period of 3 to 10 days

Management of Anesthesia Depending on the size of the defect, the anticipated difficulty with closure, and the preoperative status of the infant, continuous invasive monitoring of arterial and central venous pressure may be indicated Vigorous fluid therapy (10 to 100 ml/kg/hr) is often critical to maintain perfusion and hemodynamic stability, but glucose should be delivered at maintenance rates (3 to 4 mg/kg/min) Heat loss should be minimized and a warm environment maintained Neuromuscular blockade is essential to maximize efforts for primary closure of the abdomen The inspired oxygen concentration must be adjusted to maintain oxygen saturation between 95% and 97% while recognizing that the physiologic range of Pao₂ in newborns is 50 to 70 mm Hg Nitrous oxide may increase bowel distention and is therefore avoided

A variety of combinations of intravenous drugs and volatile anesthetics can provide adequate surgical conditions High doses of opioids are acceptable because except in infants with a small defect, mechanical ventilation of the lungs must be maintained postoperatively Prolonged postoperative total parenteral nutrition(TPN) is generally required, especially in infants with a large omphalocele or gastroschisis, so appropriate intravenous access should be established in the operating room to facilitate early postoperative nutritional support

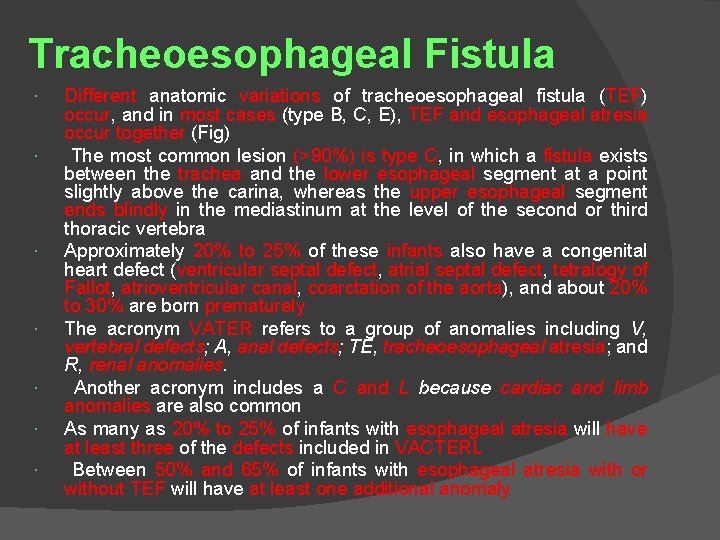
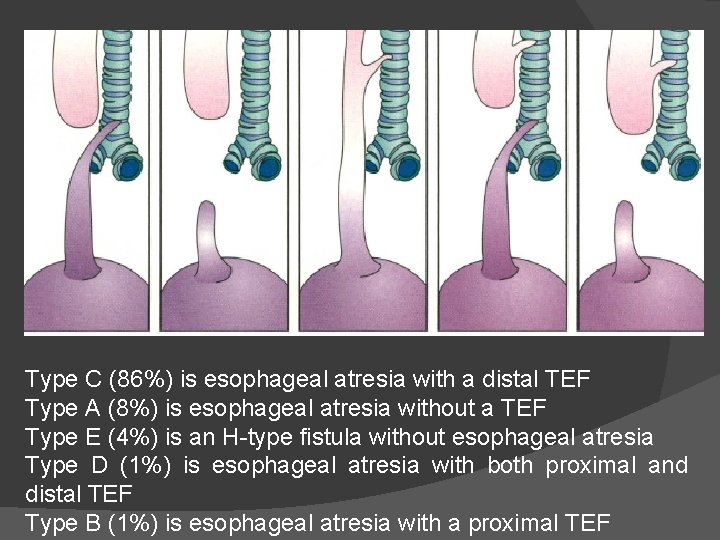
Tracheoesophageal Fistula Different anatomic variations of tracheoesophageal fistula (TEF) occur, and in most cases (type B, C, E), TEF and esophageal atresia occur together (Fig) The most common lesion (>90%) is type C, in which a fistula exists between the trachea and the lower esophageal segment at a point slightly above the carina, whereas the upper esophageal segment ends blindly in the mediastinum at the level of the second or third thoracic vertebra Approximately 20% to 25% of these infants also have a congenital heart defect (ventricular septal defect, atrial septal defect, tetralogy of Fallot, atrioventricular canal, coarctation of the aorta), and about 20% to 30% are born prematurely The acronym VATER refers to a group of anomalies including V, vertebral defects; A, anal defects; TE, tracheoesophageal atresia; and R, renal anomalies. Another acronym includes a C and L because cardiac and limb anomalies are also common As many as 20% to 25% of infants with esophageal atresia will have at least three of the defects included in VACTERL Between 50% and 65% of infants with esophageal atresia with or without TEF will have at least one additional anomaly

Type C (86%) is esophageal atresia with a distal TEF Type A (8%) is esophageal atresia without a TEF Type E (4%) is an H-type fistula without esophageal atresia Type D (1%) is esophageal atresia with both proximal and distal TEF Type B (1%) is esophageal atresia with a proximal TEF

TREATMENT Surgical ligation of a TEF is performed promptly Optimally, the surgical repair can be accomplished as a one-stage procedure in which the fistula is ligated and the esophagus is primarily anastomosed In infants with significant associated anomalies or sepsis, a thoracotomy may be considered too risky, and instead, a palliative procedure (gastrostomy) is performed under local or general anesthesia and the definitive repair performed within 24 to 72 hours, when the extent of other anomalies is defined, cardiovascular stability is established, and a clear surgical plan has been defined Often, the gastrostomy tube is kept patent to decompress the stomach and minimize regurgitation into the lungs

Interventions to Protect the Lungs from Aspiration in the Presence of a Tracheoesophageal Fistula(TEF) Avoidance of feedings Upright positioning of the infant to decrease the likelihood of gastroesophageal reflux Intermittent suctioning of the upper blind esophageal pouch Antibiotic therapy and physiotherapy if pneumonia is diagnosed

MANAGEMENTOF ANESTHESIA Awake tracheal intubation is likely to be considered the safest approach to secure the airway in infants with TEF It allows appropriate positioning of the endotracheal tube without positive-pressure ventilation, as well as minimizes the risk for gastric distention from inspired gases passing through the fistula during positive -pressure ventilation of the newborn's lungs Titrating small doses of fentanyl (0. 2 to 0. 5 чg/kg IV) or morphine (0. 02 to 0. 05 mg/kg IV) before tracheal intubation may be helpful, but this must be considered from the perspective of the infant's clinical status at the time of the procedure An alternative to awake tracheal intubation is inhalation induction of anesthesia (after pharyngeal suctioning), with or without a neuromuscular blocking drug, plus gentle positive-pressure ventilation of the infants' lungs. After the endotracheal tube is in place, end-tidal carbon dioxide and oxygen saturation are monitored and the stomach and chest auscultated to ensure that the lungs are adequately ventilated and the stomach is not being inflated with inspired gas Blood clots or secretions may block the endotracheal tube, and thus frequent suctioning is usually required

After the TEF is ligated, a catheter passed through the nose or mouth by the anesthesiologist into the blind upper pouch identifies the upper esophageal structure The surgeon passes a catheter into the lower part of the esophagus, and the anastomosis is fashioned over the catheter When the anastomosis is complete, the catheter is withdrawn to just above the suture line, and the proximal end of the catheter is marked at the mouth The distance from the mouth to the distal tip is noted Only catheters of this length or shorter should be used to suction postoperatively Some term infants can be extubated after simple ligation of a TEF, but more often postoperative ventilation is maintained for at least 24 to 48 hours Tracheomalacia or a defective tracheal wall at the site of the fistula is common and can predispose to collapse of the airway during spontaneous ventilation Most surgeons recommend that ventilation with a mask and bag be avoided for at least several days after an esophageal anastomosis

PERSISTENT EFFECTS AFTER SURGICAL REPAIR TEF cannot be considered a simple anatomic problem cured by a surgical intervention Many patients have anatomic narrowing of the esophagus at the site of anastomosis or ligation of the fistula, and this narrowing may progress to a severe stricture Esophageal dysmotility and reflux are common and may also lead to esophageal stricture Recurrent upper and lower respiratory infections occur in 35% to 75% of patients Pulmonary function studies 7 to 18 years after repair show a high incidence of obstructive and restrictive forms of lung disease

Congenital Diaphragmatic Hernia Congenital diaphragmatic hernia (CDH) is a defect in the diaphragm that develops early in gestation and is accompanied by extrusion of intra-abdominal organs into the thoracic cavity and other associated abnormalities(Table &Fig) In many infants with CDH, the herniated abdominal viscera that occupy the thoracic cage interfere with development of the lungs Typically, some degree of pulmonary hypoplasia is present on the ipsilateral side, but often also on the contralateral side

Abnormalities That May Be Associated with Congenital Diaphragmatic Hernia Bilateral lung hypoplasia (varying degrees) Pulmonary hypertension Pulmonary arteriolar hyperreactivity and arteriolar reactivity Congenital anomalies Cardiac Chromosomal


CLINICAL MANIFESTATIONS The classic triad of CDH consists of cyanosis, dyspnea, and apparent dextrocardia Physical examination reveals a scaphoid abdomen, bulging chest, decreased breath sounds, distant or right-displaced heart sounds, and bowel sounds in the chest Radiographs may show a bowel gas pattern in the chest, mediastinal shift, and minimal lung tissue at the left costophrenic sulcus

TREATMENT The goal of initial management of CDH is to avoid a surgical intervention when the infant is hypoxic and acidotic Instead, medical management is directed to stabilizing the infant's cardiorespiratory status by improving oxygenation, correcting metabolic acidosis, reducing the right-to-left shunting, and increasing pulmonary perfusion Positive-pressure ventilation with a facemask is particularly risky for infants with CDH because attempting to expand the noncompliant lungs may distend the stomach and intestines, which are in the left thoracic cavity, and thereby further decrease chest compliance Early tracheal intubation and decompression of the stomach are important initial steps to prevent further compression of the infant's lungs by the displaced abdominal viscera In the early neonatal period, some infants exhibit a honeymoon period characterized by adequate oxygenation and ventilation with minimal ventilatory support

However, this period is often followed by a sudden and often unexplained return to a state of persistent pulmonary hypertension (persistent fetal circulation) and clinical deterioration (acidosis, arterial hypoxemia, hypercapnia, pulmonary hypertension, and right -to-left shunting through the foramen ovale and ductus arteriosus) Efforts at manipulating pulmonary vascular resistance (pharmacologic means or hyperventilation, or both) are not predictably successful If conventional mechanical ventilation of the infant's lungs is not effective, a trial of high-frequency oscillatory support may be considered Otherapeutic interventions to consider include the administration of inhaled nitric oxide and extracorporeal membrane oxygenation (ECMO) to achieve cardiorespiratory stability before surgery If an infant does require either nitric oxide or ECMO, surgery is likely to be performed in the neonatal intensive care unit(NICU) so that these therapies can be maintained

MANAGEMENT OF ANESTHESIA Usually, surgical repair is approached through an abdominal incision, but a transthoracic or thoracoabdominal approach is also possible With rare exception, infants with a moderate or large CDH require ventilatory support preoperatively, and most receive neuromuscular blockade The goals of ventilation in the operating room (optimize PH and pulmonary blood flow with minimal barotrauma) are the same as preoperatively Hyperventilation of the infant's lungs is reserved to treat acute episodes of pulmonary hypertension If sudden deterioration in ventilation or hemodynamic status (or both) occurs, pulmonary hypertension must be quickly differentiated from a contralateral pneumothorax because treatment of pneumothorax is needle thoracostomy and chest tube placement rather than hyperventilation

The selection of specific anesthetic drugs is logically based on cardiorespiratory status, the site of surgical repair (neonatal intensive care nursery or operating room), and the plans for intraoperative ventilatory support Nitrous oxide is avoided in infants with CDH because most require high inspired oxygen concentrations and nitrous oxide can diffuse inside the viscera and exaggerate lung compression If an anesthesia machine is available, a low concentration of a volatile anesthetic can be administered and the dose increased if the newborn is hemodynamically stable In most cases, opioids (usually intravenous fentanyl) are administered an opioid infusion is continued into the postoperative period

Myelomeningocele The primary defect in a myelomeningocele is localized failure of the neural tube to close The lesion appears most often in the lumbar area as a sac on the back of the infant In addition, because the vertebral arches in the area fail to fuse or are totally absent, the spinal canal is widened

CLINICAL MANIFESTATIONS The clinical manifestations of myelomeningocele relate to the level of neural tube involvement, theexistence ofotherneurologicanomalies, and the development of hydrocephalus (develops in about 90% of infants with thoracolumbar, or lumbosacral lesions) In addition, the Arnold-Chiari malformation is identified in most infants with this lesion

TREATMENT Treatment of myelomeningocele is based on the predicted prognosis In some infants, supportive care is an appropriate level of conservative intervention In many infants, prenatal diagnosis introduces the consideration of fetal treatment or cesarean section, or both, to avoid trauma during spontaneous vaginal delivery In most cases of thoracolumbar, and lumbosacral lesions, surgical intervention is initiated in the first 24 hours of life Early surgical treatment decreases the incidence of infection and further neurologic injury The main anesthetic considerations are related to avoiding injury during induction and tracheal intubation and meticulous attention to ventilation in the prone position The infant can be supported on "bolsters" while supine during induction and again when positioned prone for surgical treatment Placement of a ventriculoperitoneal shunt is generally performed in a separate surgery The need for frequent surgical interventions is the basis for initiating latex precautions from the neonatal period in the hope of minimizing the risk for development of latex allergy

Pyloric Stenosis The abnormal anatomy leading to pyloric stenos. Is is thickened circular smooth muscle of the pylorus The gastric outlet gradually becomes obstructed over the first days to weeks of life and leads to projectile, nonbilious vomiting and failure to thrive, with body weight less than birth weight at 2 to 3 weeks of age The pathognomonic physical finding is an olive-sized mass in the upper left to midportion of the abdomen The condition is more common in white, first-born males The most significant preoperative considerations are fluid and electrolyte imbalance and a full stomach Because of recurrent vomiting of acidic gastric fluid, hypokalemic hypochloremic metabolic alkalosis and dehydration may develop Administration of intravenous fluids allows rehydration and correction of the metabolic abnormalities within 6 to 12 hours in most infants At that point, surgical treatment is indicated

MANAGEMENTOF ANESTHESIA Some anesthesiologist prefer to consider an infant as having a full stomach and recommend a rapid-sequence induction of anesthesia after emptying the stomach with a nasogastric tube Others suggest that the stomach empties spontaneously and believe that a inhalational or intravenous (not necessarily rapid -sequence) induction of anesthesia is acceptable A history of a barium swallow may affect the decision regarding the technique for induction of anesthesia. However, sonography and not a barium swallow is the most frequent technique for confirming the diagnosis of pyloric stenosis Although the surgical procedure is a simple pyloromyotomy that takes only about 15 minutes, the current trend is to perform a laparoscopic procedure to minimize recovery time and the likelihood of ileus The infant may be able to eat within several hours of surgery, and discharge is often possible within about 12 hours

COMPLICATING ISSUES IN THE CLINICAL MANAGEMENT OF PEDIATRIC PATIENTS

Upper Respiratory Infection URI has diffuse effects on the respiratory epithelium, mucociliary function, and airway reactivity These effects combine to provide the potential for an increased risk for anesthesia in specific clinical settings If the planned surgical procedure is short and airway support is restricted to the use of a facemask, the risk for an adverse respiratory event is minimal If an endotracheal tube is required, the risk for an adverse respiratory event is increased (up to 10 - fold) over that in an infant without a URI whose trachea is not intubated An LMA seems to be associated with risks midway between those associated with a facemask and those with an endotracheal tube Younger age plus a URI seems to be associated with an increased risk from anesthesia

URIs develop recurrently in 1 - to 6 -year-olds, and if reactive airways accompany the infection, the effect on the airway persists for 2 to 6 weeks The decision to cancel surgery on a child with an uncomplicated URI always requires assessment from the viewpoint of a specific patient and family, a specific procedure, and a specific surgeon A strict protocol for when to cancel surgery is impractical The patient's age, medical and anesthetic history, current physical examination, planned surgery (placement of tympanostomy tubes versus surgery for craniofacial repair), and anticipated postoperative care (need for mechanical ventilatory support) must be analyzed Ultimately, the preoperative evaluation must weigh the inconvenience of rescheduling against ignoring possible risks If the decision is to proceed with elective surgery, the infant should be considered to have reactive airways

OUTPATIENT SURGERY Inguinal herniorrhaphy, hypospadias repair, and various orthopedic procedures are commonly performed in young children as outpatient surgery The use of an LMA plus a caudal block (1 mg/kg of 0. 125% to 0. 25% bupivacaine or ropivacaine) provides excellent postoperative pain control while decreasing intraoperative anesthetic requirements The more dilute local anesthetic solution may be of benefit to ambulatory children by avoiding significant motor blockade Laparoscopic inguinal hernia repair, unlike an open repair, generally requires endotracheal intubation, and caudal anesthesia is not needed.

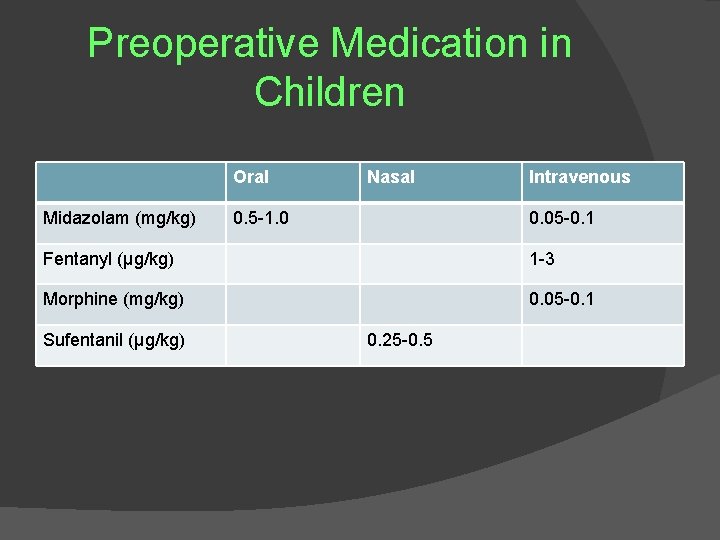
Preoperative Medication in Children Oral Midazolam (mg/kg) Nasal 0. 5 -1. 0 Intravenous 0. 05 -0. 1 Fentanyl (µg/kg) 1 -3 Morphine (mg/kg) 0. 05 -0. 1 Sufentanil (µg/kg) 0. 25 -0. 5

EX-PREMATURE INFANT Despite many studies dealing with postoperative apnea in expremature infants, a precise protocol defining the need for postoperative monitoring cannot be developed “ This may reflect the difficulty in defining an ex-premature infant For example, a 36 -week-gestation infant who had an unremarkable 3 -day stay in the intensive care nursery is distinct from a 28 -weekgestation infant who had NEC, respiratory insufficiency, and chronic lung disease and is continuing oxygen therapy and diuretics at home. Furthermore, although apnea is rare after about 48 weeks‘ postconceptual age, the incidence is not zero Even the definition of apnea varies from study to study Thus, the decision to admit an ex-premature infant after surgery requires individual assessment of the patient, consideration of the surgical procedure, and the time when the surgery is completed (early morning, late afternoon) The most conservative approach is to admit every expremature infant who is younger than 60 weeks' postconceptual age, but this is impractical in many cases

In addition to the potential risk for postoperative apnea, ex-premature infants commonly have chronic lung conditions characterized by reactive airways disease and abnormal pulmonary function for the first decade of life, if not longer Developmental delay may affect plans for premedication and may contribute to unexpected responses to sedatives and anesthetics Hepatic and renal dysfunction is not uncommon
 Reza entezari maleki
Reza entezari maleki Zaira masood
Zaira masood Chris chien
Chris chien Masood mansoori
Masood mansoori Introduction to pediatrics
Introduction to pediatrics Radial pulse range
Radial pulse range Vital signs ranges
Vital signs ranges Types of jaundice
Types of jaundice Jacket restraint indications
Jacket restraint indications Pediatric iv medication administration guidelines
Pediatric iv medication administration guidelines Chest pain in pediatrics
Chest pain in pediatrics What are normal vital signs
What are normal vital signs Peter lascarides
Peter lascarides Differential diagnosis of jaundice in pediatrics
Differential diagnosis of jaundice in pediatrics Modern concept of paediatric nursing
Modern concept of paediatric nursing Introduction modern concept of child care
Introduction modern concept of child care Surgery shelf percentiles
Surgery shelf percentiles Chest pain in pediatrics
Chest pain in pediatrics Bristol pediatrics
Bristol pediatrics Im injection sites and volumes pediatrics
Im injection sites and volumes pediatrics Trends in pediatric nursing 2020
Trends in pediatric nursing 2020 Nec diagnosis
Nec diagnosis Duke pediatrics durham nc
Duke pediatrics durham nc Holzer pediatrics
Holzer pediatrics Vancouver clinic pediatrics
Vancouver clinic pediatrics Equipment used in pediatrics
Equipment used in pediatrics Lsu pediatrics residency
Lsu pediatrics residency Himalaya vigorcare price
Himalaya vigorcare price Nelson pediatrics
Nelson pediatrics Practical approach pediatrics
Practical approach pediatrics Holistic pediatrics
Holistic pediatrics River valley pediatrics
River valley pediatrics Warm chain in pediatrics
Warm chain in pediatrics Disease xxx
Disease xxx Nelson pediatrics
Nelson pediatrics Nelson pediatrics
Nelson pediatrics Combivent para bebes
Combivent para bebes Peds vital signs chart
Peds vital signs chart Tlc pediatrics flint
Tlc pediatrics flint Nn pediatrics
Nn pediatrics Mummy restraint in pediatrics
Mummy restraint in pediatrics Carrie tingley pediatrics
Carrie tingley pediatrics Nidra poller
Nidra poller Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok
Tidbok Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Hur skriver man en tes
Hur skriver man en tes Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Tryck formel
Tryck formel Offentlig förvaltning
Offentlig förvaltning Lyckans minut erik lindorm analys
Lyckans minut erik lindorm analys Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Plats för toran ark
Plats för toran ark Treserva lathund
Treserva lathund Fimbrietratt
Fimbrietratt Claes martinsson
Claes martinsson Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Lågenergihus nyproduktion
Lågenergihus nyproduktion Mat för idrottare
Mat för idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Hur ser ett referat ut
Hur ser ett referat ut Redogör för vad psykologi är
Redogör för vad psykologi är Borstål, egenskaper
Borstål, egenskaper Atmosfr
Atmosfr Borra hål för knoppar
Borra hål för knoppar Orubbliga rättigheter
Orubbliga rättigheter Varians
Varians Tack för att ni har lyssnat
Tack för att ni har lyssnat Rita perspektiv
Rita perspektiv Informationskartläggning
Informationskartläggning Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling dos
Toppslätskivling dos Mästar lärling modellen
Mästar lärling modellen Egg för emanuel
Egg för emanuel