Double Replacement Reactions Predicting Solubility High Solubility means

Double Replacement Reactions

Predicting Solubility High Solubility means that a lot of compound will dissolve in water (> 0. 1 moles per 1 L) Low Solubility means very little compound will dissolve in water (< 0. 1 moles per 1 L)
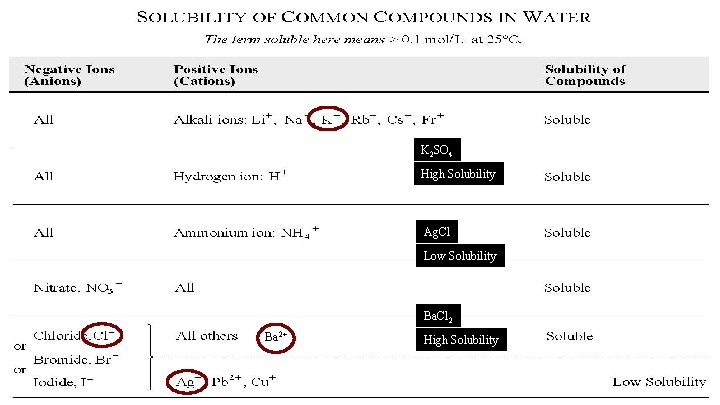
K 2 SO 4 High Solubility Ag. Cl Low Solubility Ba. Cl 2 Ba 2+ High Solubility
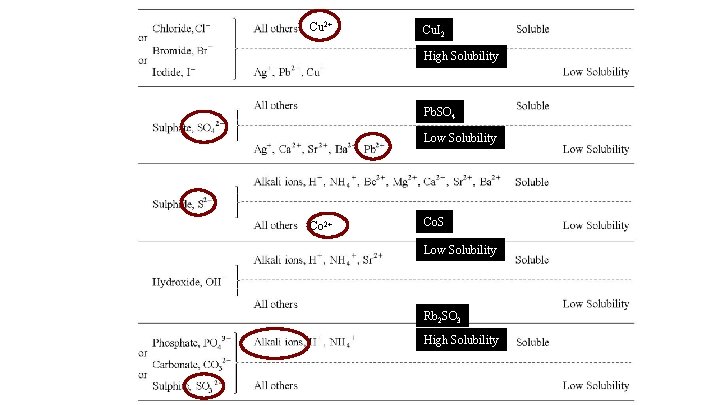
Cu 2+ Cu. I 2 High Solubility Pb. SO 4 Low Solubility Co 2+ Co. S Low Solubility Rb 2 SO 3 High Solubility
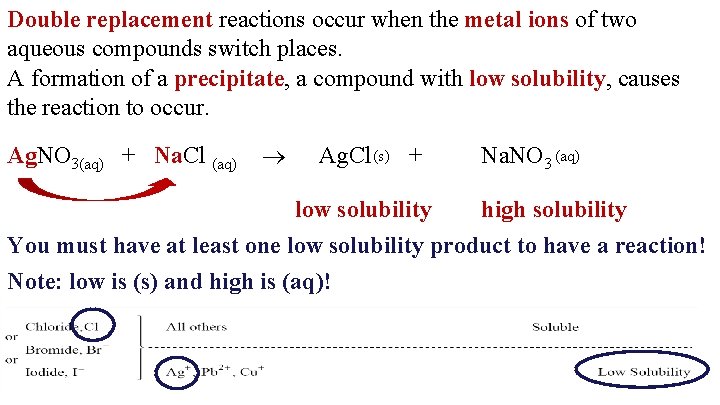
Double replacement reactions occur when the metal ions of two aqueous compounds switch places. A formation of a precipitate, a compound with low solubility, causes the reaction to occur. Ag. NO 3(aq) + Na. Cl (aq) Ag. Cl (s) + low solubility Na. NO 3 (aq) high solubility You must have at least one low solubility product to have a reaction! Note: low is (s) and high is (aq)!
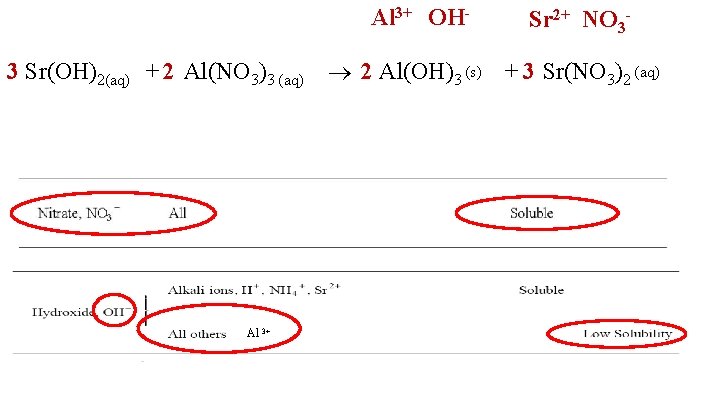
Al 3+ OH 3 Sr(OH)2(aq) + 2 Al(NO 3)3 (aq) Al 3+ Sr 2+ NO 3 - 2 Al(OH)3 (s) + 3 Sr(NO 3)2 (aq)
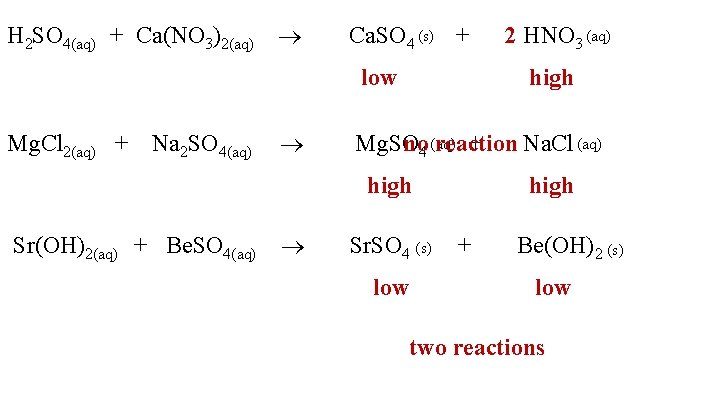
H 2 SO 4(aq) + Ca(NO 3)2(aq) Ca. SO 4 (s) + low Mg. Cl 2(aq) + Na 2 SO 4(aq) high no 4 (aq) reaction Na. Cl (aq) Mg. SO + high Sr(OH)2(aq) + Be. SO 4(aq) 2 HNO 3 (aq) Sr. SO 4 (s) low high + Be(OH)2 (s) low two reactions
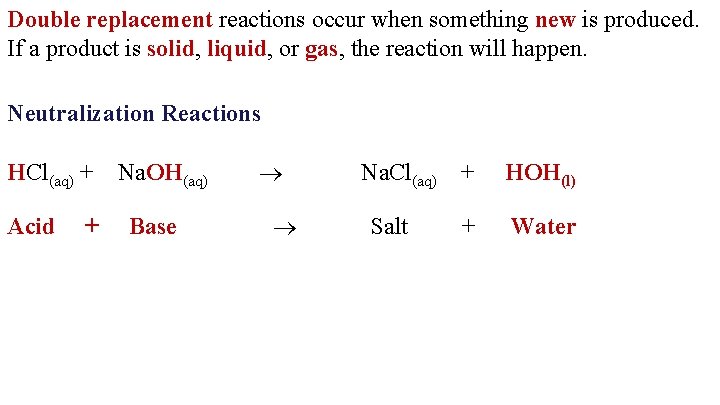
Double replacement reactions occur when something new is produced. If a product is solid, liquid, or gas, the reaction will happen. Neutralization Reactions HCl(aq) + Acid + Na. OH(aq) Base Na. Cl(aq) Salt + HOH(l) + Water
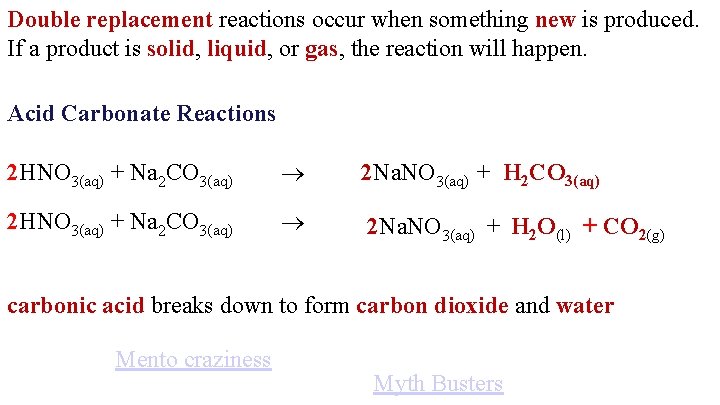
Double replacement reactions occur when something new is produced. If a product is solid, liquid, or gas, the reaction will happen. Acid Carbonate Reactions 2 HNO 3(aq) + Na 2 CO 3(aq) 2 Na. NO 3(aq) + H 2 CO 3(aq) 2 HNO 3(aq) + Na 2 CO 3(aq) 2 Na. NO 3(aq) + H 2 O(l) + CO 2(g) carbonic acid breaks down to form carbon dioxide and water Mento craziness Myth Busters
- Slides: 9