Double Replacement Reactions Exchange of ions between two

Double Replacement Reactions

• • Exchange of ions between two compounds Typically a precipitate is produced AX+BY -> AY+BX EX. Al 2(CO 3)3 + K 2 O -> Al 2 O 3 + K 2 CO 3

• Anions switch place with anions and cations switch place with cations. • Ex. Na. Cl + Ag. NO 3 -> Na. NO 3 + Ag. Cl
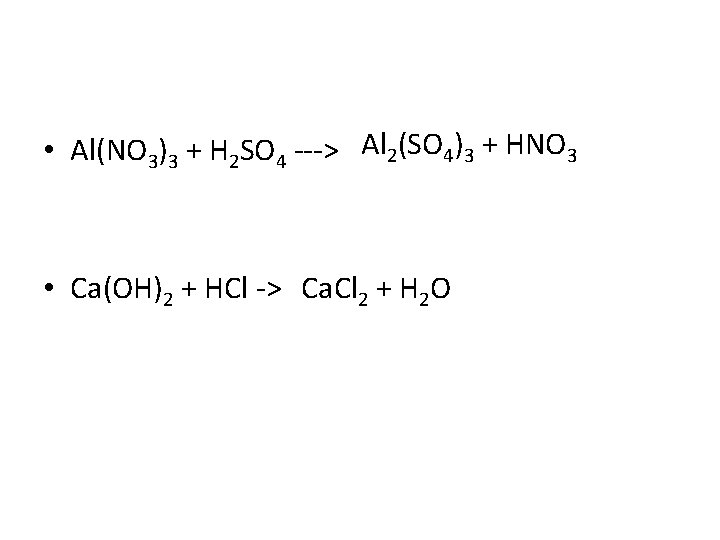
• Al(NO 3)3 + H 2 SO 4 ---> Al 2(SO 4)3 + HNO 3 • Ca(OH)2 + HCl -> Ca. Cl 2 + H 2 O

Do all reactions always occur? • No! • Must have one soluble and one insoluble compounds. • Soluble – dissolves in water, its state is aqueous • Insoluble - forms a precipitate, a solid compound
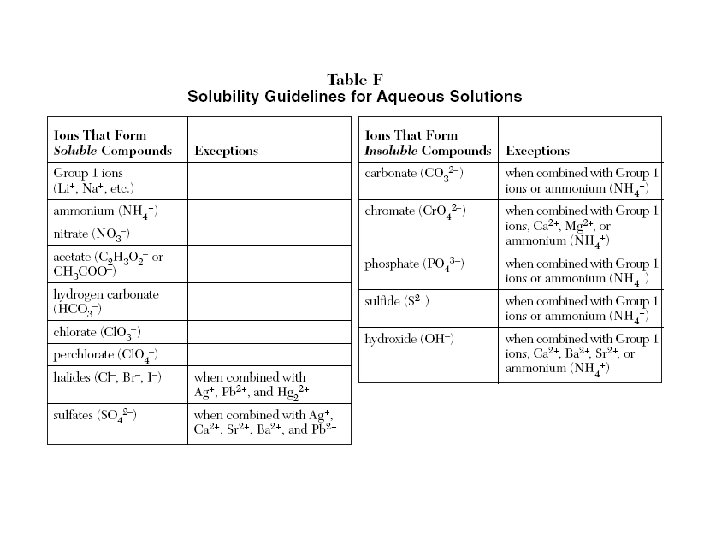

Rules • One of the products is only slightly soluble and precipitates from solution • One of the products is a gas • One product is a molecular compound such as water
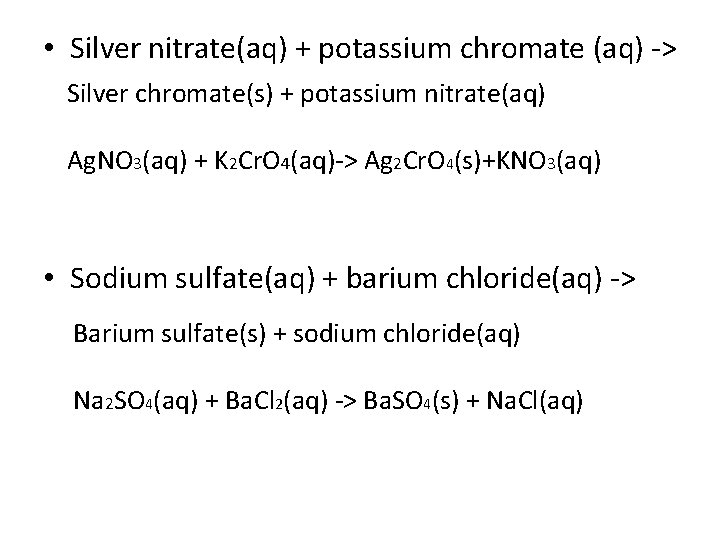
• Silver nitrate(aq) + potassium chromate (aq) -> Silver chromate(s) + potassium nitrate(aq) Ag. NO 3(aq) + K 2 Cr. O 4(aq)-> Ag 2 Cr. O 4(s)+KNO 3(aq) • Sodium sulfate(aq) + barium chloride(aq) -> Barium sulfate(s) + sodium chloride(aq) Na 2 SO 4(aq) + Ba. Cl 2(aq) -> Ba. SO 4(s) + Na. Cl(aq)

• Calcium hydroxide(aq)+hydrochloric acid(aq)-> Calcium chloride (aq) + water (aq) Ca(OH)2(aq) + HCl (aq) -> Ca. Cl 2(aq) + H 2 O • Potassium chloride(aq)+sulfuric acid(aq)-> Potassium sulfate (aq)+hydrochloric acid (aq) NO REACTION KCl(aq) + H 2 SO 4(aq) -> K 2 SO 4(aq)+HCl(aq)

• Copper sulfide(aq)+potassium chloride(aq)-> Copper chloride (aq) + potassium sulfide (aq) Cu. Cl 2(aq) + K 2 S(aq) NO REACTION

• The pancreas secretes sodium bicarbonate into the small intestine to neutralize gastric acid. The same thing happens when you have acidity and eat Tums. HCl + Na. HCO 3 → Na. Cl + H 2 CO 3 • acid soil & rain: Ca. SO 4 & H 2 O --> Ca(OH)2 (s) & H 2 SO 4
- Slides: 12