Double Replacement Lab SAFETY REVIEW MSDS INSTRUCTIONS About

Double Replacement Lab SAFETY REVIEW, MSDS, INSTRUCTIONS
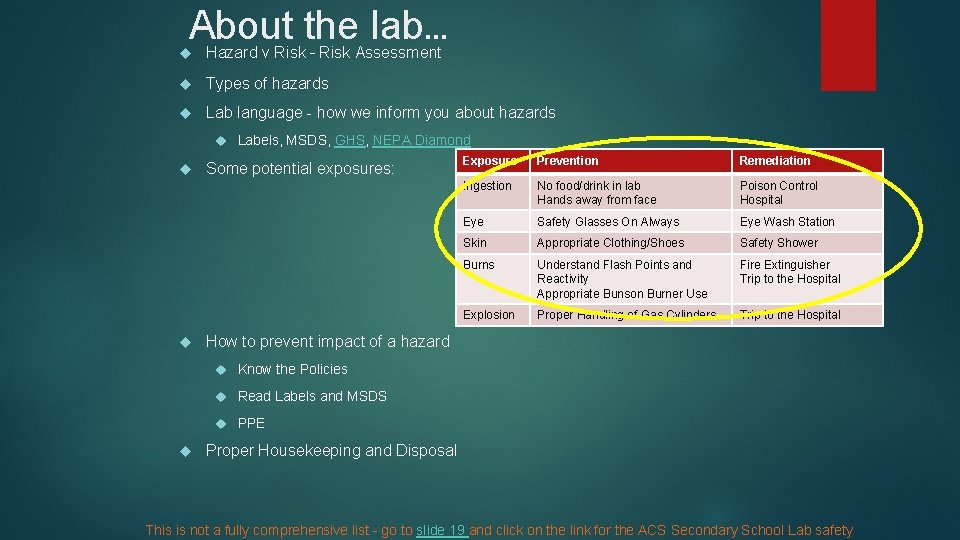
About the lab… Hazard v Risk – Risk Assessment Types of hazards Lab language - how we inform you about hazards Labels, MSDS, GHS, NEPA Diamond Some potential exposures: Exposure Prevention Remediation Ingestion No food/drink in lab Hands away from face Poison Control Hospital Eye Safety Glasses On Always Eye Wash Station Skin Appropriate Clothing/Shoes Safety Shower Burns Understand Flash Points and Reactivity Appropriate Bunson Burner Use Fire Extinguisher Trip to the Hospital Explosion Proper Handling of Gas Cylinders Trip to the Hospital How to prevent impact of a hazard Know the Policies Read Labels and MSDS PPE Proper Housekeeping and Disposal This is not a fully comprehensive list – go to slide 19 and click on the link for the ACS Secondary School Lab safety
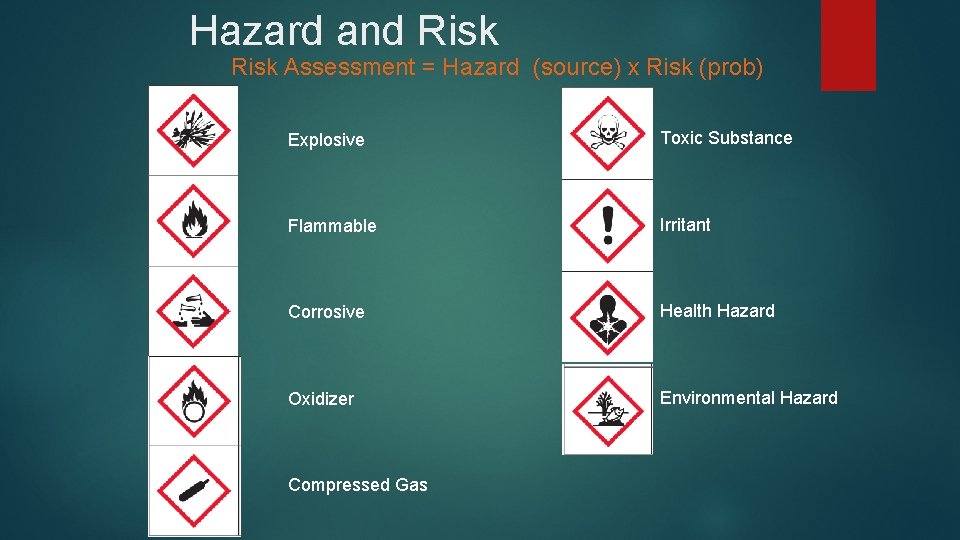
Hazard and Risk Assessment = Hazard (source) x Risk (prob) Explosive Toxic Substance Flammable Irritant Corrosive Health Hazard Oxidizer Environmental Hazard Compressed Gas
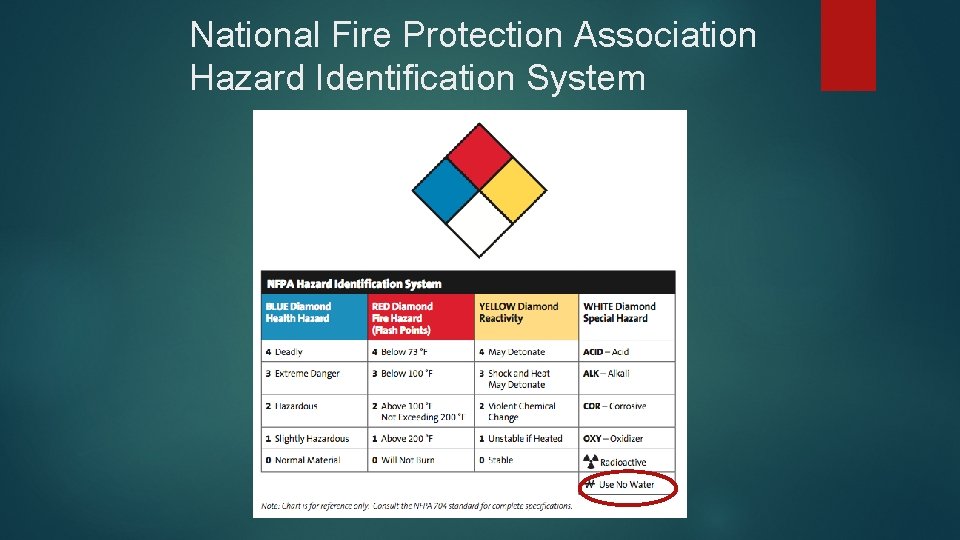
National Fire Protection Association Hazard Identification System

NOT in Progar. Chem Lab Cell Phones Cell phones may be out, but must be SCREEN DOWN. No phone calls or texts or surfing. You may listen to music during individual or group work. No texting apps or phone calls. One warning – second time I take the phone to be returned end of class. Having anything other than your notebook & unit packet out. Completing work for other classes.

Health and Reactivity Risks

Lab Execution Instructions 1. There will be 7 Lab teams. I will read your team assignment. Pick a partner. 2. Put your safety glasses and lab aprons on before entering the lab 3. Take a PEN, lab sheet with you to the lab 4. Execute the lab: 1. Put a transparency sheet on top of the lab sheet side 1. 2. Obtain the first two chemicals at the first intersection eg Ag. NO 3 and Na 2 CO 3. Place 2 drops of Na 2 CO 3 on to the transparency where it says Observation. Document the observation on your lab sheet. Complete all of the reactions using Na 2 CO 3 and then move to the next solution, continuing to document your observations. 3. After you have completed the first side of the sheet. Pull the white sheet out from underneath so you can see the reactions that you might have missed due to the white color of the sheet behind the transparence. Any you may have missed will be easier to see against the black benchtop. 4. Rinse the transparency off into the sink. 5. Begin again on side 2 and repeat 1 -4 6. Now identify the substances you have created by formula and name on your lab sheet. 7. Turn in your lab sheet 8. Dry any water left on your lab bench and return to your seat.
- Slides: 7