Double Replacement Acid Carbonate Reactions Write dissociation equations

Double Replacement & Acid Carbonate Reactions

Write dissociation equations for the following ionic compounds to show they dissolve in water. Cu. SO 4 (s) Cu 2+ (aq) Fe. Cl 3(s) Fe 3+(aq) + Fe 2(HPO 4)3 (s) + SO 42 - (aq) 3 Cl- (aq) 2 Fe 3+(aq) + 3 HPO 42 -(aq)
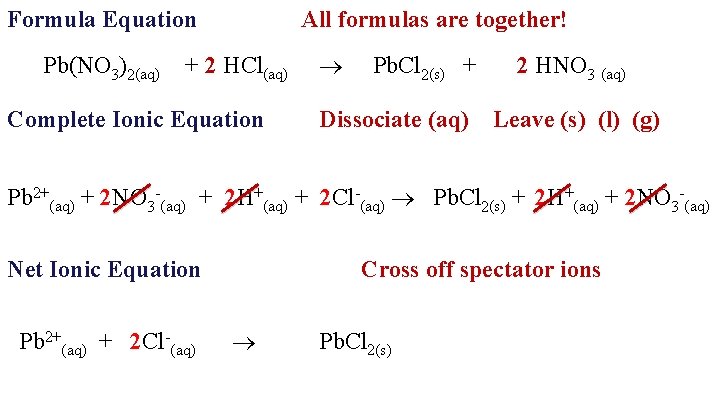
Formula Equation Pb(NO 3)2(aq) All formulas are together! + 2 HCl(aq) Complete Ionic Equation Pb. Cl 2(s) + 2 HNO 3 (aq) Dissociate (aq) Leave (s) (l) (g) Pb 2+(aq) + 2 NO 3 -(aq) + 2 H+(aq) + 2 Cl-(aq) Pb. Cl 2(s) + 2 H+(aq) + 2 NO 3 -(aq) Net Ionic Equation Pb 2+(aq) + 2 Cl-(aq) Cross off spectator ions Pb. Cl 2(s)
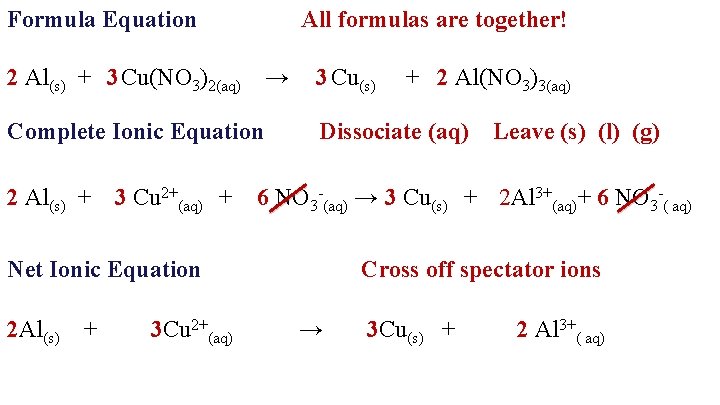
Formula Equation All formulas are together! 2 Al(s) + 3 Cu(NO 3)2(aq) → Complete Ionic Equation 2 Al(s) + 3 Cu 2+(aq) + 3 Cu(s) Dissociate (aq) Leave (s) (l) (g) 6 NO 3 -(aq) → 3 Cu(s) + 2 Al 3+(aq)+ 6 NO 3 -( aq) Net Ionic Equation 2 Al(s) + 3 Cu 2+(aq) + 2 Al(NO 3)3(aq) Cross off spectator ions → 3 Cu(s) + 2 Al 3+( aq)
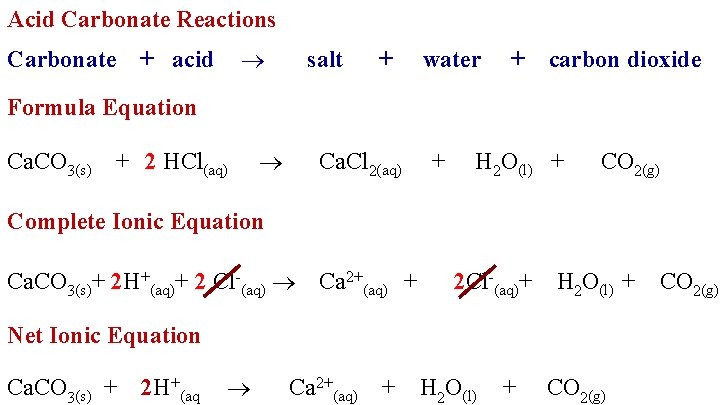
Acid Carbonate Reactions Carbonate + acid salt + water + carbon dioxide Formula Equation Ca. CO 3(s) + 2 HCl(aq) Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) Complete Ionic Equation Ca. CO 3(s)+ 2 H+(aq)+ 2 Cl-(aq) Ca 2+(aq) + 2 Cl-(aq)+ H 2 O(l) + Net Ionic Equation Ca. CO 3(s) + 2 H+(aq Ca 2+(aq) + H 2 O(l) + CO 2(g)
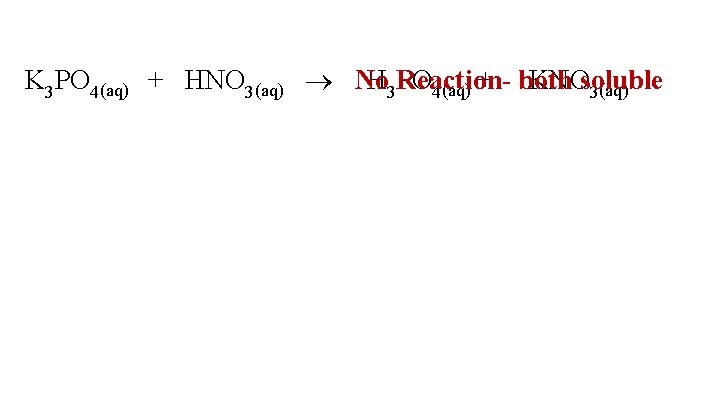
K 3 PO 4(aq) + HNO 3(aq) No H 3 Reaction. PO 4(aq) + both KNOsoluble 3(aq)
- Slides: 6