Double Displacement Reactions Special Cases Double Displacement Reactions

Double Displacement Reactions Special Cases
Double Displacement Reactions that Form a Gas �Sometimes, the production of a gas, instead of a precipitate, indicates that a double displacement reaction has occurred. �When gas is formed, it is usually because one of the products quickly decomposes into water and a gas.

Formation of Carbon Dioxide Gas CO 2(g) �If during the double displacement reaction, carbonic acid (H 2 CO 3(aq)) is produced, then it will quickly decompose creating carbon dioxide gas and water
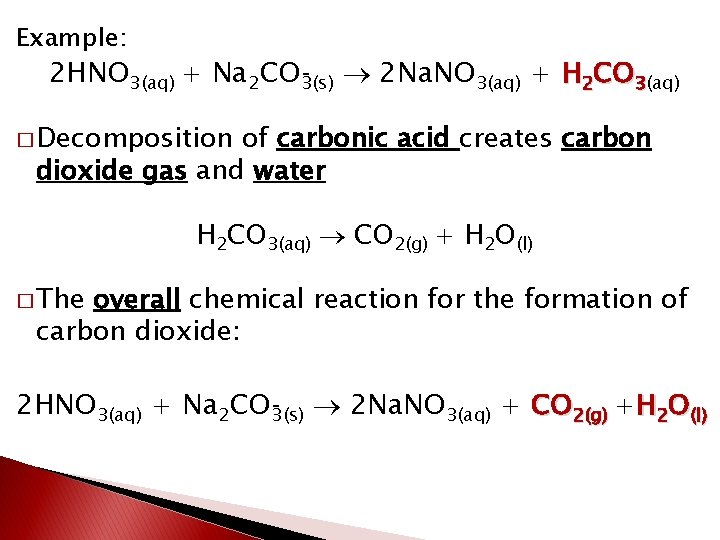
Example: 2 HNO 3(aq) + Na 2 CO 3(s) 2 Na. NO 3(aq) + H 2 CO 3(aq) � Decomposition of carbonic acid creates carbon dioxide gas and water H 2 CO 3(aq) CO 2(g) + H 2 O(l) � The overall chemical reaction for the formation of carbon dioxide: 2 HNO 3(aq) + Na 2 CO 3(s) 2 Na. NO 3(aq) + CO 2(g) +H 2 O(l)

�Example 1: Write a balanced chemical equation for the double displacement reaction between calcium carbonate, Ca. CO 3(aq), and hydrochloric acid, HCl (aq).
Formation of Ammonia Gas NH 3(g) �If during the double displacement reaction, ammonium hydroxide (NH 4 OH(aq)) is produced, then it will quickly decompose creating ammonia gas (NH 3(g)) and water
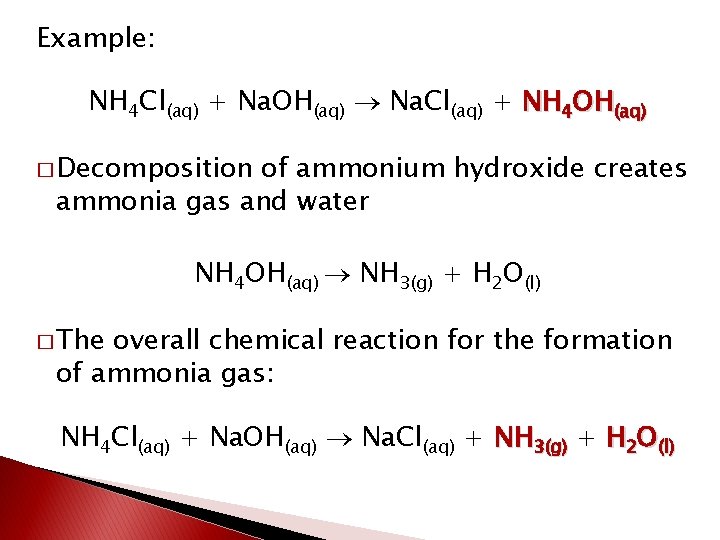
Example: NH 4 Cl(aq) + Na. OH(aq) Na. Cl(aq) + NH 4 OH(aq) � Decomposition of ammonium hydroxide creates ammonia gas and water NH 4 OH(aq) NH 3(g) + H 2 O(l) � The overall chemical reaction for the formation of ammonia gas: NH 4 Cl(aq) + Na. OH(aq) Na. Cl(aq) + NH 3(g) + H 2 O(l)

�Example 2: Write a balanced chemical equation for the double displacement reaction between ammonium chloride, NH 4 Cl(aq), and beryllium hydroxide, Be(OH)2(aq).
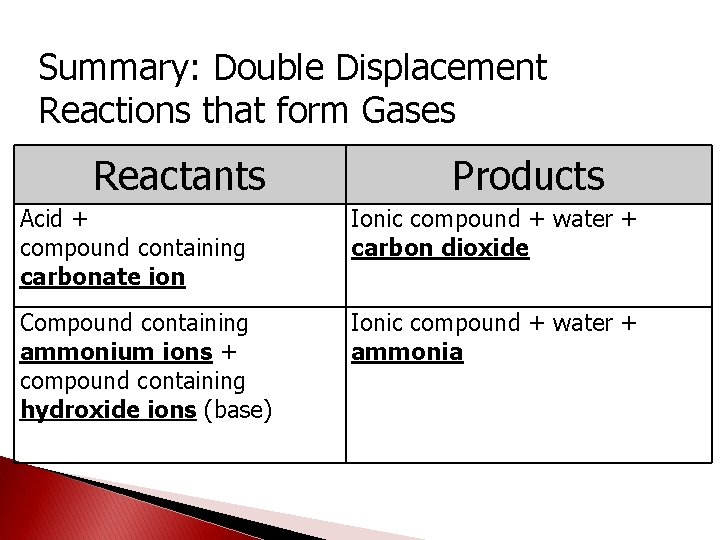
Summary: Double Displacement Reactions that form Gases Reactants Products Acid + compound containing carbonate ion Ionic compound + water + carbon dioxide Compound containing ammonium ions + compound containing hydroxide ions (base) Ionic compound + water + ammonia
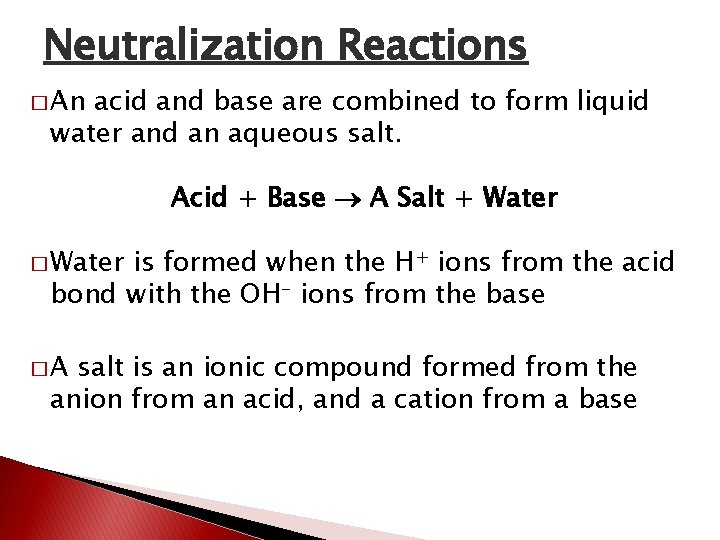
Neutralization Reactions � An acid and base are combined to form liquid water and an aqueous salt. Acid + Base A Salt + Water � Water is formed when the H+ ions from the acid bond with the OH- ions from the base �A salt is an ionic compound formed from the anion from an acid, and a cation from a base

�Example 3: Write the balanced chemical equation for the neutralization reaction between nitric acid and sodium hydroxide.

- Slides: 12