Double Displacement Reactions Lesson Outline Double Displacement Reactions
Double Displacement Reactions

Lesson Outline Double Displacement Reactions that Form a Precipitate Acid and Base Double Displacement Reactions that Form Water
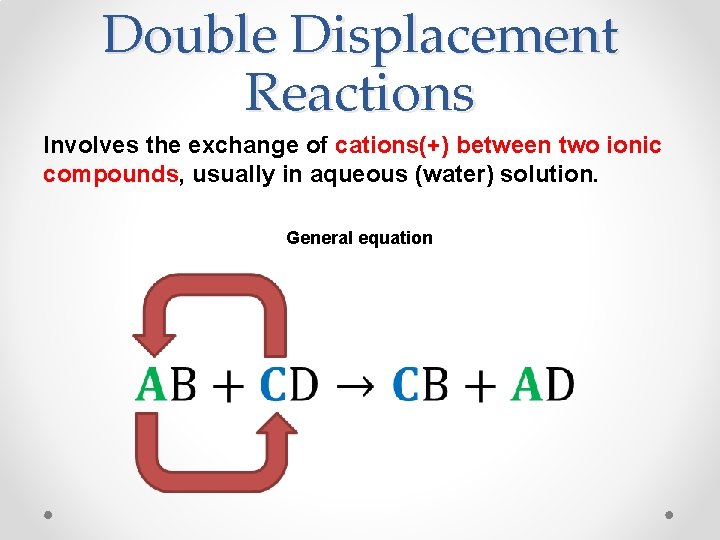
Double Displacement Reactions Involves the exchange of cations(+) between two ionic compounds, usually in aqueous (water) solution. General equation

Precipitate An insoluble solid that is formed by a chemical reaction of two soluble compounds in water. DD Rxn must form a precipitate or no reaction will occur
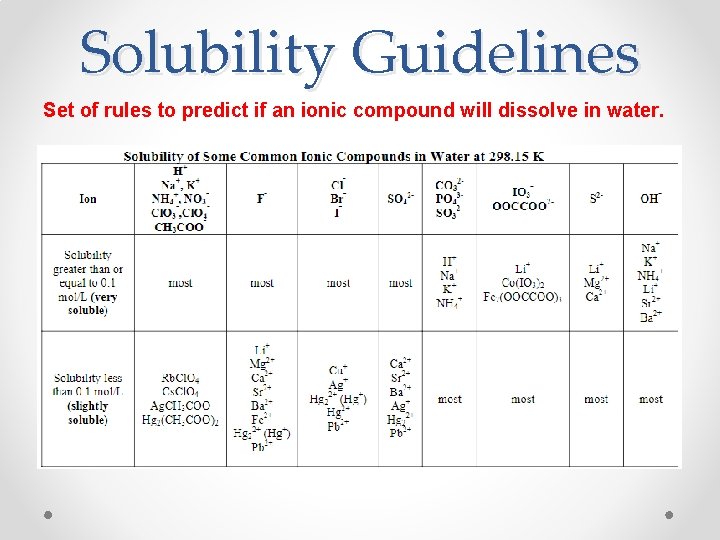
Solubility Guidelines Set of rules to predict if an ionic compound will dissolve in water.

DD Rxn Forming a Precipitate How to complete the reaction: 1. Switch the cations of the two reactant form the products. 1. Using the solubility rules, determine if one of the products will form a precipitate. no precipitate = no reaction (NR) 2. Use the zero sum rule to determine the compound ratio of each product. 3. Balance the equation.

DD Rxn Forming a Precipitate Whiteboard activity Fe. Cl 3(aq) + Na 2 SO 4(aq) Silver Nitrate + Potassium Phosphate Calcium acetate + Sodium Carbonate

Mystery Solution Game Choose one of the following reactants to indicate which beaker contains the solution of dissolved Na. Cl. 6 reactants located at side bench table 1. Hypothesize which reactant will indicate the beaker containing dissolved Na. Cl solution. 2. Write the complete equation of this reaction.

Testing Predictions Sodium Chloride + Silver Nitrate Iron (III) Chloride + Sodium Sulfate Sodium Bi. Phosphate + Silver Nitrate

Testing Predictions 1. 2. 3. 4. Everyone obtain safety goggles. Obtain 1 spot plate per table. Add the first reactant in 1 well of the spot plate. Add the second reactant to the same well and observe for a precipitate. 5. Repeat the procedure for the remaining two reactions. 6. Clean all tests tubes and return them.

Acid and Base Neutralization Reactions Acid + Base Salt + water HNO 3(aq) + Na. OH(aq) Na. NO 3(aq) + H 2 O(l) 1. Recognize its an Acid/Base Rxn, switch the cations and form water. 2. Use the Zero Sum Rule to determine the products. 3. Balance the chemical equation.
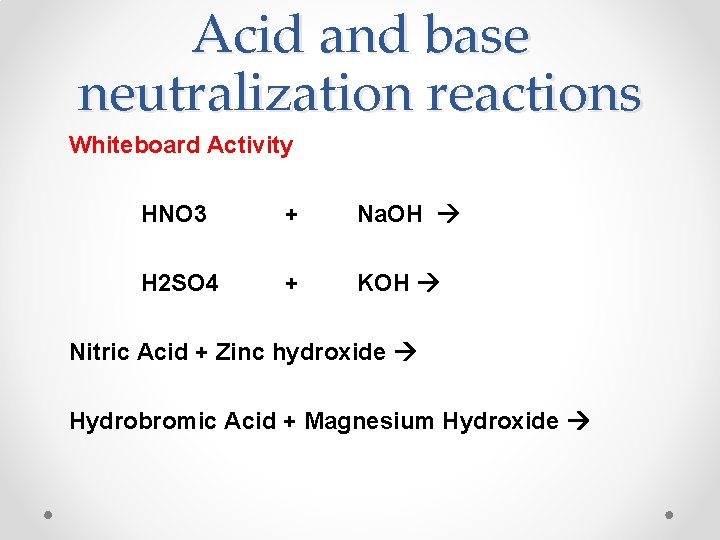
Acid and base neutralization reactions Whiteboard Activity HNO 3 + Na. OH H 2 SO 4 + KOH Nitric Acid + Zinc hydroxide Hydrobromic Acid + Magnesium Hydroxide

Looking Forward Combustion Reactions & Reaction Review Chemical Reactions Quiz
- Slides: 13