Double Beam spectrometer Provides a signal that is

Double Beam spectrometer • Provides a signal that is largely free of drift in the source and detector without requiring really expensive components • Beam alternates very fast between sample and reference cells. • Don’t need to keep zeroing
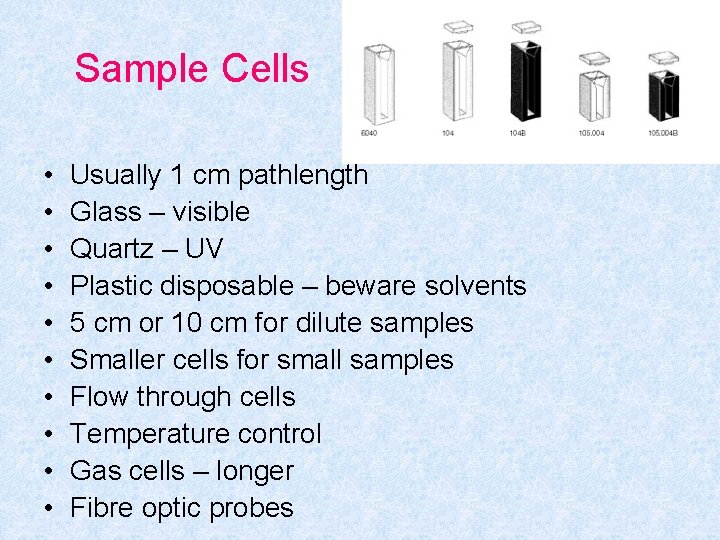
Sample Cells • • • Usually 1 cm pathlength Glass – visible Quartz – UV Plastic disposable – beware solvents 5 cm or 10 cm for dilute samples Smaller cells for small samples Flow through cells Temperature control Gas cells – longer Fibre optic probes
Fibre Optics • Fibres of glass, usually about 120 µm in diameter.
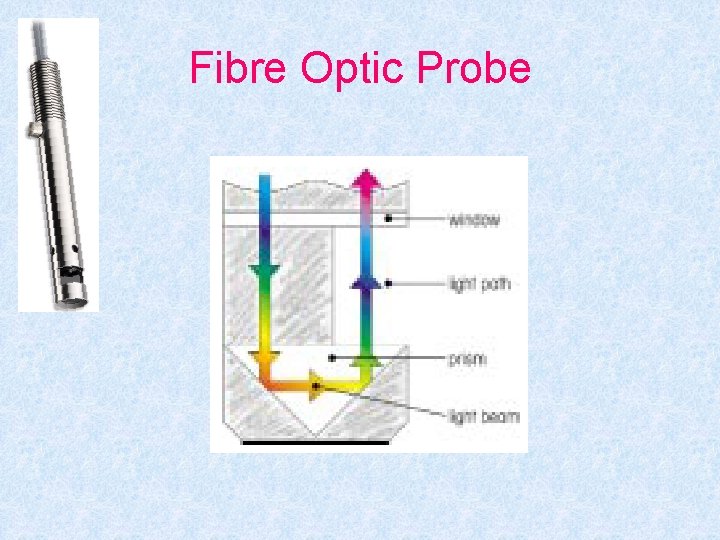
Fibre Optic Probe

Derivative Spectroscopy • Can determine flat maxima more precisely • Isolate shoulders • Distinguish weak signals from background
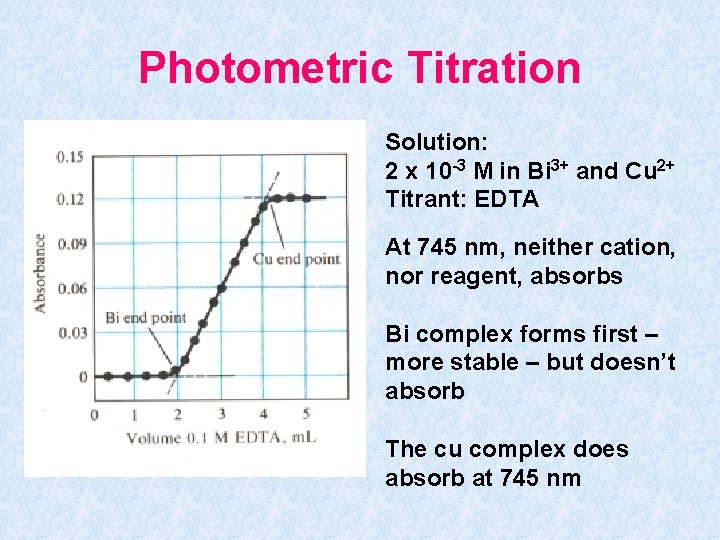
Photometric Titration Solution: 2 x 10 -3 M in Bi 3+ and Cu 2+ Titrant: EDTA At 745 nm, neither cation, nor reagent, absorbs Bi complex forms first – more stable – but doesn’t absorb The cu complex does absorb at 745 nm

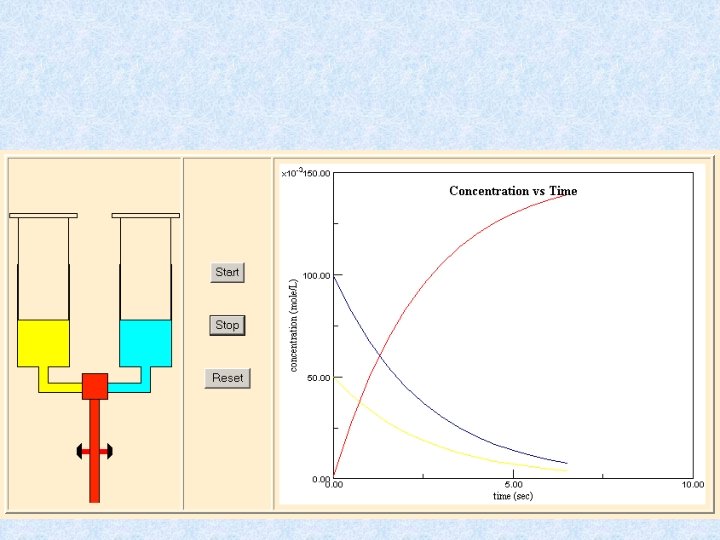
Reaction rates • Following enzyme kinetics • Determine enzymes • Determine substrates

Stop Flow Methods • • For fast reactions Two syringes driven at same rate Solutions flow into mixing chamber When plunger hits stop, measurement starts • Generally measure initial rates of reactions
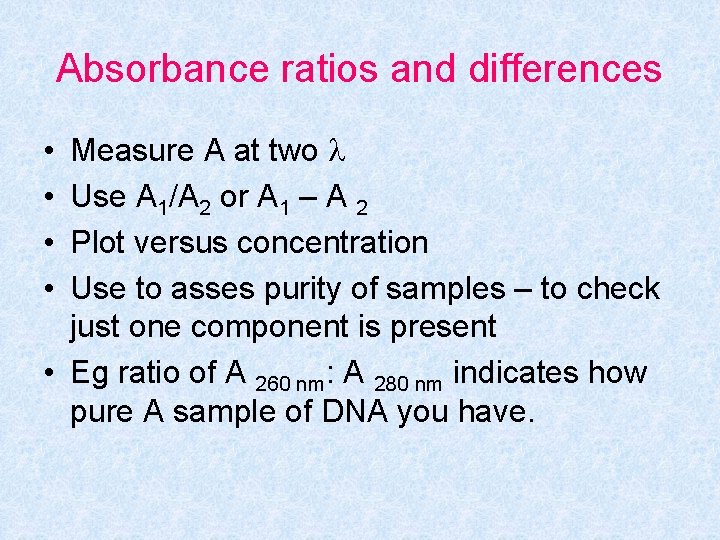
Absorbance ratios and differences Measure A at two Use A 1/A 2 or A 1 – A 2 Plot versus concentration Use to asses purity of samples – to check just one component is present • Eg ratio of A 260 nm: A 280 nm indicates how pure A sample of DNA you have. • •

Applications • • Metal ion analysis eg iron II or III React with ligands to get intense colours Reduce Fe III with hydroxylamine or hydroquinone etc • Can extract the complexes into isoamyl alcohol for a cleanup/preconcentration step • 0. 1 - 0. 005 µg/m. L are typical LOD’s

Organic/Biologicals • • Most common application Many absorb strongly May need to derivatize eg alcohol with phenyl isocyanate to give alkyl carbamates – 280 nm • Free amino acids react with ninhydrin – blue/purple – 575 nm – aa analyzers

Automated clinical methods • Many samples/hour • Expensive to buy • Can run many samples unattended

Centrifugal Analysis • • Combines robotic pipettors Centrifuge Spectrophotometer Computer • Increases sample throughput • Reduces volume of sample and reagents
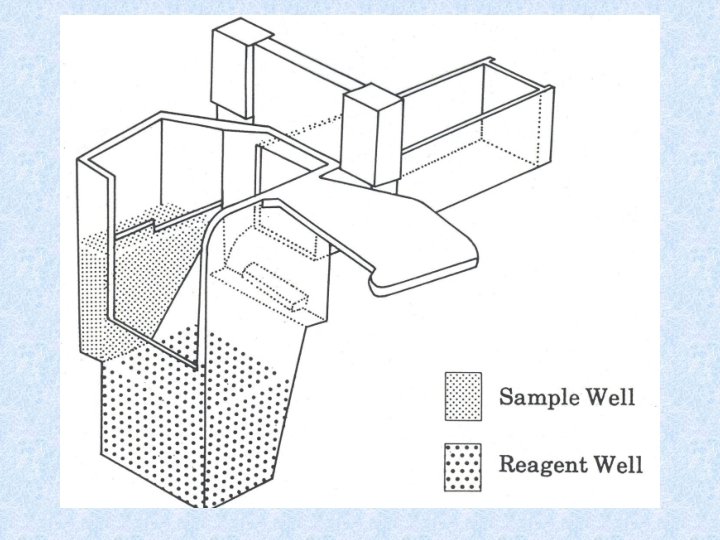

• • • Eliminates chemistry changeover time No set-up equilibrium time Used for water quality measurements Based on standard procedures Liquids are dispensed into separate compartments attached to the cuvettes • Cuvettes are round a rotor. When rotor is spun, reagents are propelled into cuvettes. • All reactions start together – good for kinetics

• Samples and standards are mixed and run in parallel. • Identical conditions are ensured for all cuvettes • Can analyze 110 samples/hour • Rotor spins at 2000 revolutions /min • Get an average of ~ 7 readings/sample

Water Pollution Analysis • • Molybdenum blue method (NH 4)3 P (Mo 3 O 10)4 yellow Reduce with hydroquinone, Sn II or Fe II Get a polymer of Mo of different oxidation states • Not stoichiometrically well-defined but is blue • As, Si interfere – so they can also be determined this way
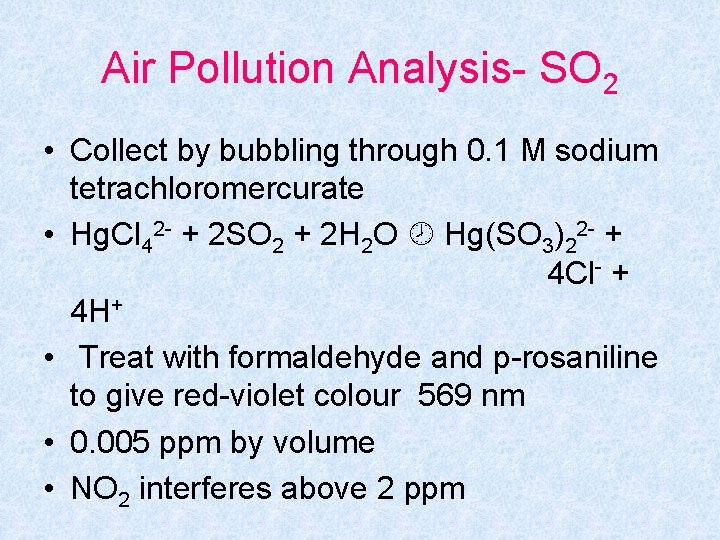
Air Pollution Analysis- SO 2 • Collect by bubbling through 0. 1 M sodium tetrachloromercurate • Hg. Cl 42 - + 2 SO 2 + 2 H 2 O Hg(SO 3)22 - + 4 Cl- + 4 H+ • Treat with formaldehyde and p-rosaniline to give red-violet colour 569 nm • 0. 005 ppm by volume • NO 2 interferes above 2 ppm

Advantages Visible • Less interferences • Often higher molar absorptivities
- Slides: 24