Dosage Regimen Design One of the practical applications







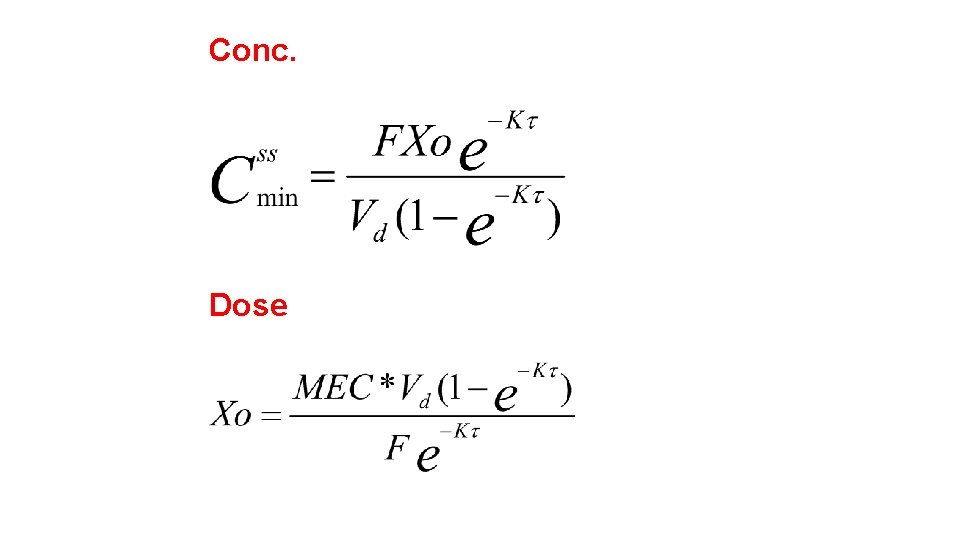

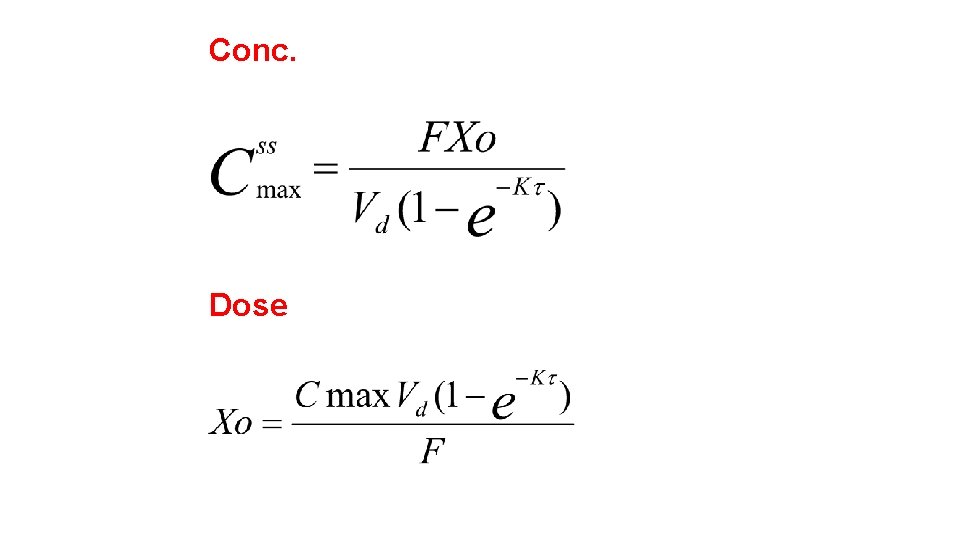












- Slides: 50

Dosage Regimen Design One of the practical applications of pharmacokinetics is the calculation of dose size and dosing intervals for drugs in patients with and without renal failure. The purpose of a dosage regimen design is, for the drug-naive patient, to initiate therapy for multiple dosing. Since there is no drug in the body, mean literature pharmacokinetic parameters are used. The mean pharmacokinetic parameters should result at steady state in blood levels close to the desired ones.

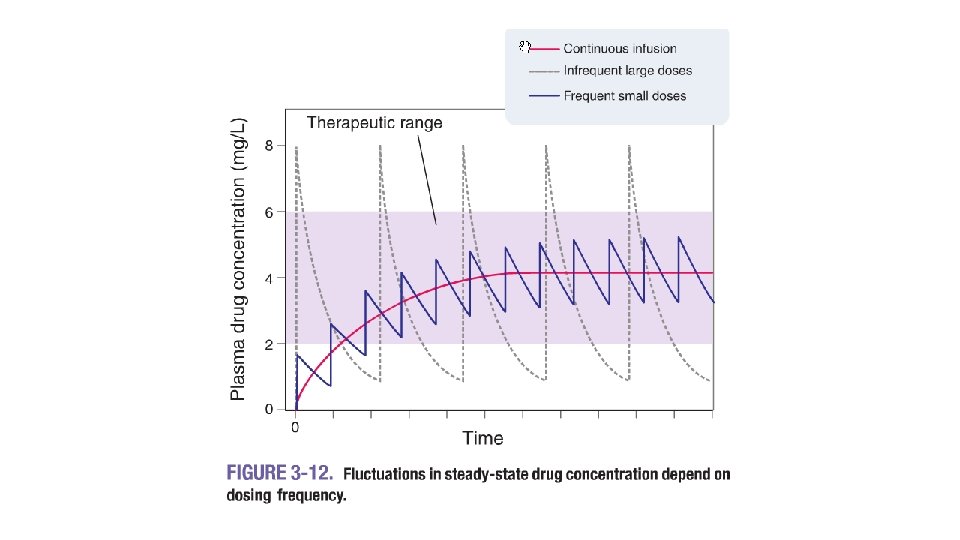
objective • The objective is to maintain a minimum inhibitory concentration (MIC), or a minimum effective concentration (MEC) or • to obtain a desired peak or • mean steady state concentration. Calculations are based on the equations of drug accumulation for multiple dosing with fixed dose sizes and fixed dosing intervals as discussed in "Pharmacokinetics of Multiple Dosing. "

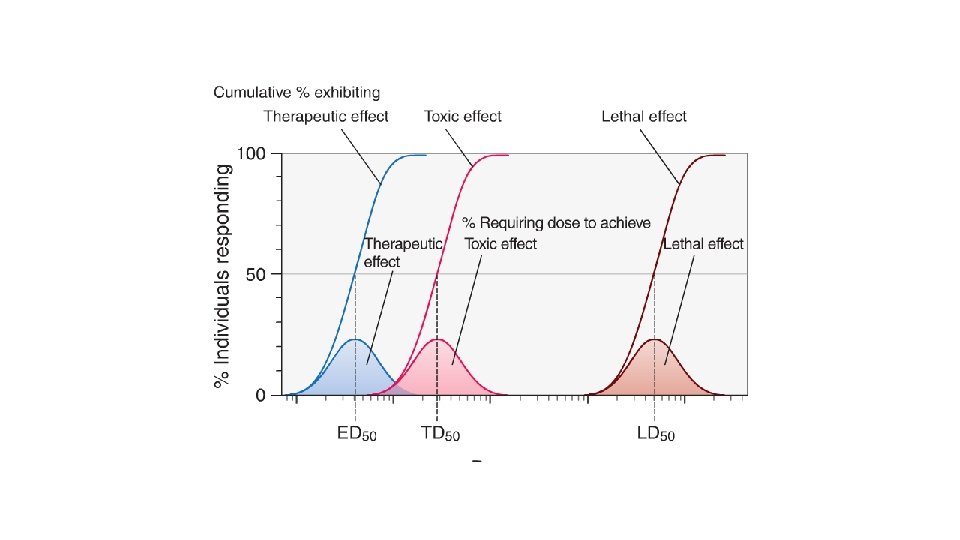
Therapeutic Range This concept is commonly misunderstood. Many inexperienced users of therapeutic drug concentration monitoring assume that therapeutic range for most drugs has been well defined from carefully controlled clinical trials. Another common misconception is that concentrations in therapeutic range will result in the desired clinical response.

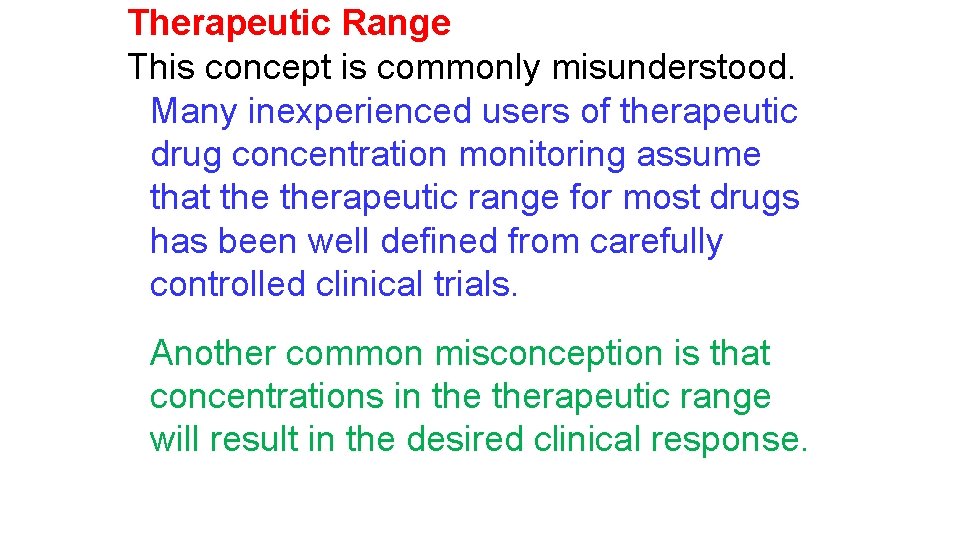
In general, a therapeutic range should never be considered in absolute terms, as it represents no more than a combination of probability charts. In other words, a therapeutic range is a range of drug concentrations within which the probability of the desired clinical response is relatively high and the probability of unacceptable toxicity is relatively low.



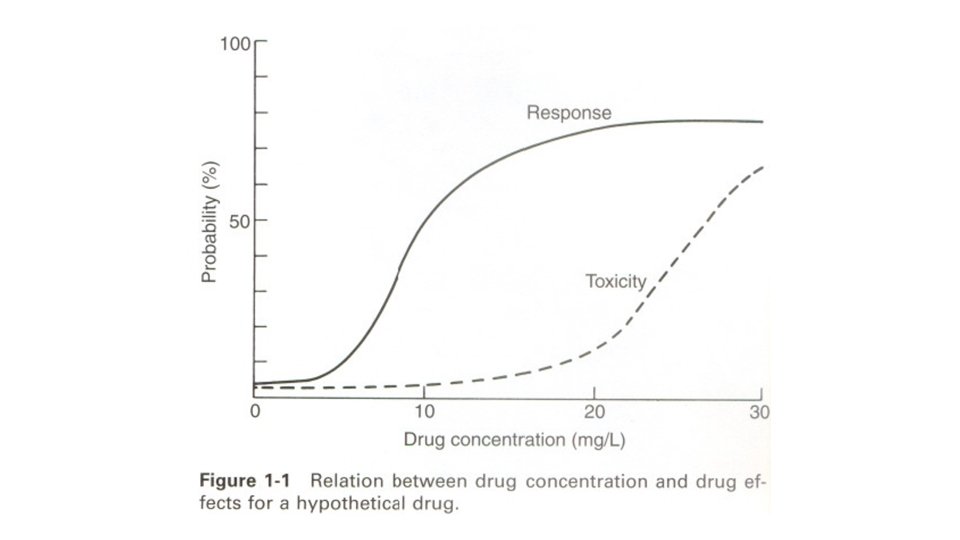
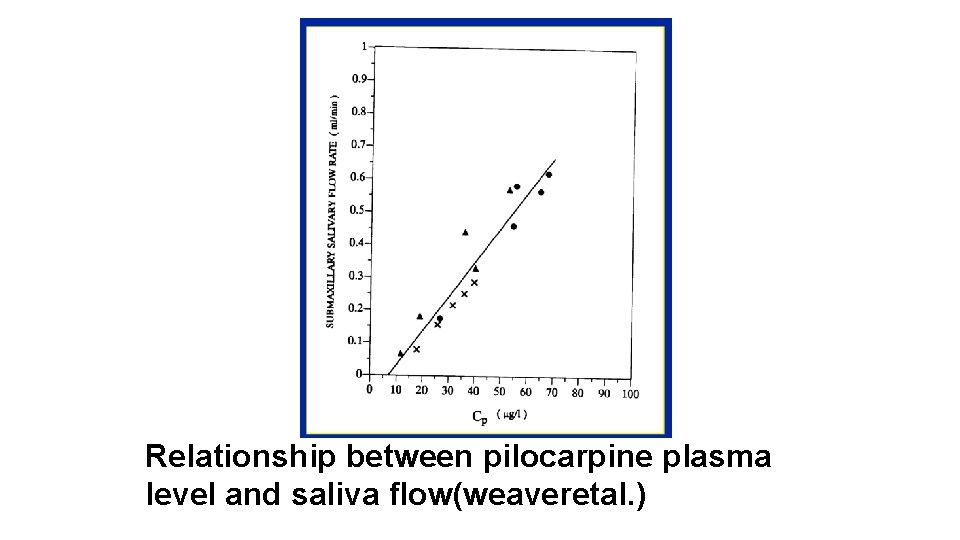
Unfortunately, concentration-effect charts such as those shown in Figures 1 -1 and 1 -2, based on large numbers of prospectively studied patients, do not exist for many drugs. Moreover, with most drugs there are discrete subpopulations (because of disease, age, concurrent therapy, inheritance and so forth) for whom concentration-effect relationships differ from the norm. The process of selecting the most appropriate dosage regimen to achieve concentrations in a relatively narrow range may be complicated by unpredictable intrapatient and interpatient variability in the drug's pharmacokinetics.

Although a single "best" approach to using drug concentrations does not exist for every drug, it is imperative to realize that without a systematic approach to therapeutic drug concentration monitoring, drug concentrations may be uninterpretable, unhelpful, and potentially harmful. It thus becomes essential to recognize the key elements of clinical pharmacokinetics and pharmacodynamics, and to develop strategies to perform and use them most effectively.

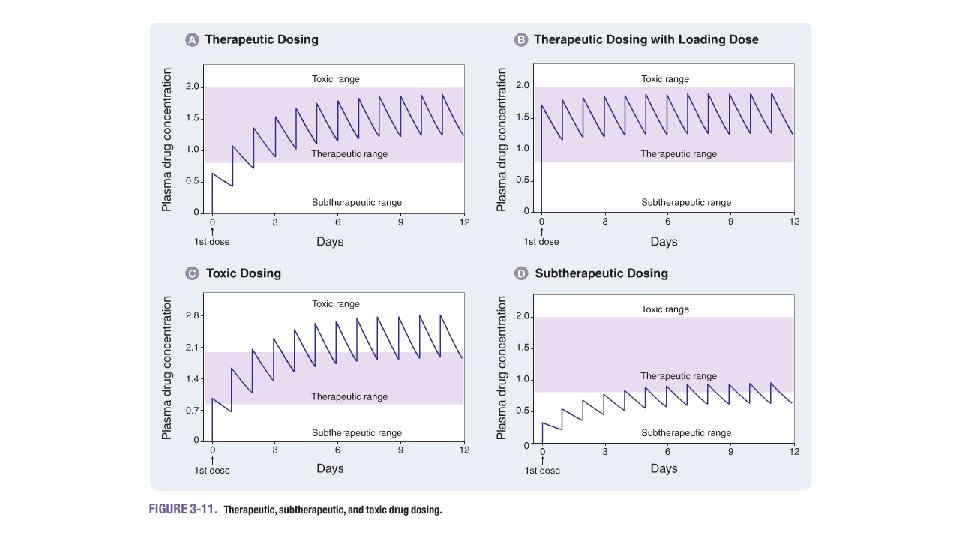
Assumptions • The equations for determination of dose sizes and dosing intervals are based on the open one -compartment model. Therefore, one must recognize the implications, assumptions, and limitations. • The following consideration especially must be kept in mind: It is assumed that all pharmacokinetic parameters remain constant during the course of therapy once a dosage regimen has been determined. In case one or more of the factors change, the once established dosage regimen is no longer valid.




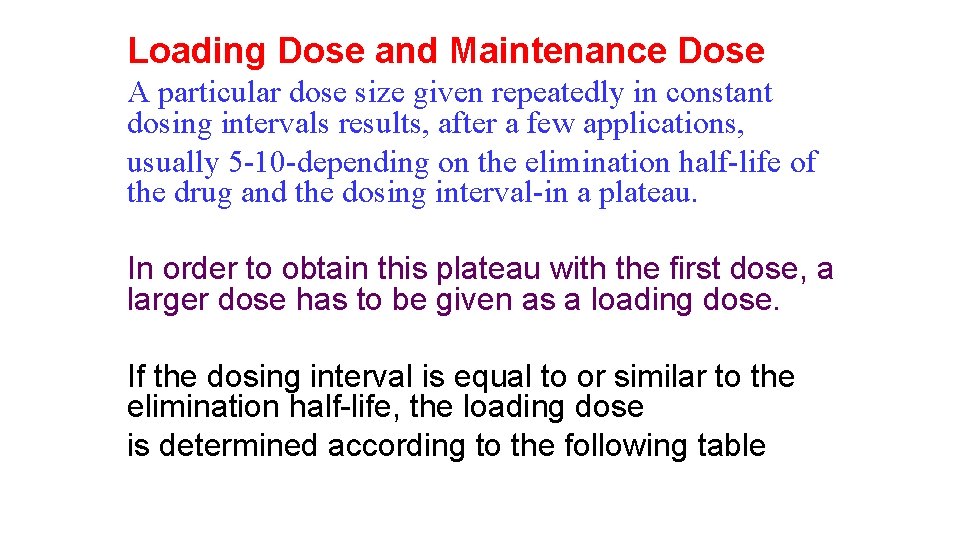
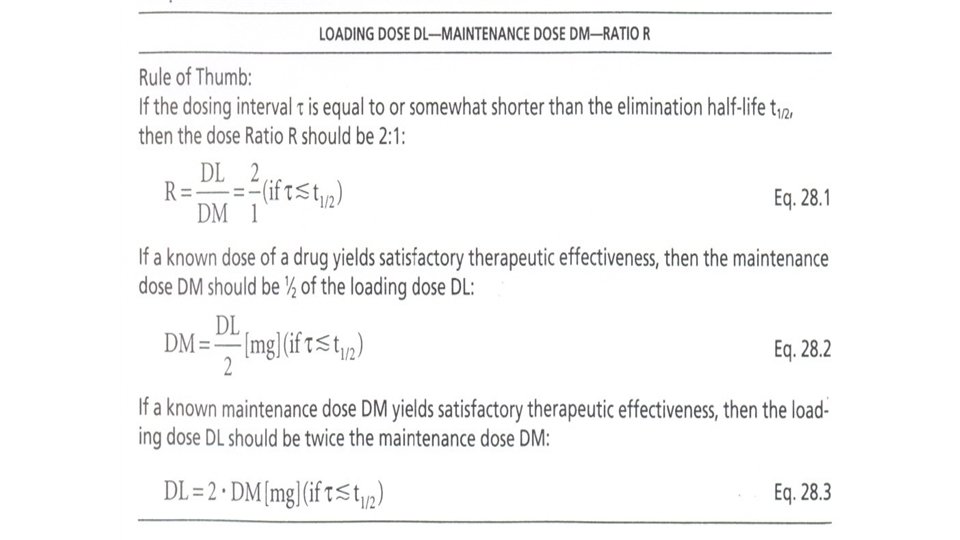
Loading Dose and Maintenance Dose A particular dose size given repeatedly in constant dosing intervals results, after a few applications, usually 5 -10 -depending on the elimination half-life of the drug and the dosing interval-in a plateau. In order to obtain this plateau with the first dose, a larger dose has to be given as a loading dose. If the dosing interval is equal to or similar to the elimination half-life, the loading dose is determined according to the following table


MAXIMUM DOSE AND MAXIMUM DOSING INTERVAL Based on the MEC and MTC, one can estimate the maximum maintenance dose that can be administered and the longest interval between two administrations as follows

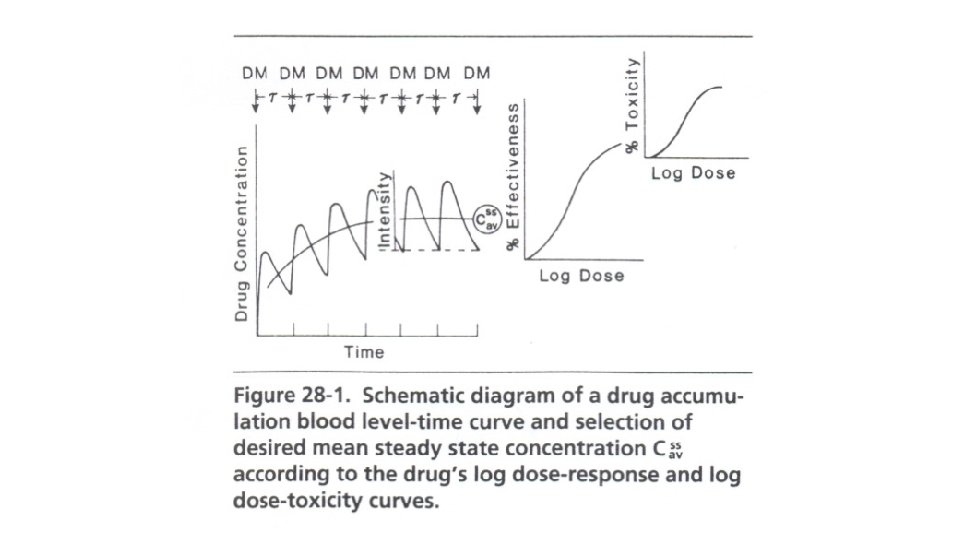
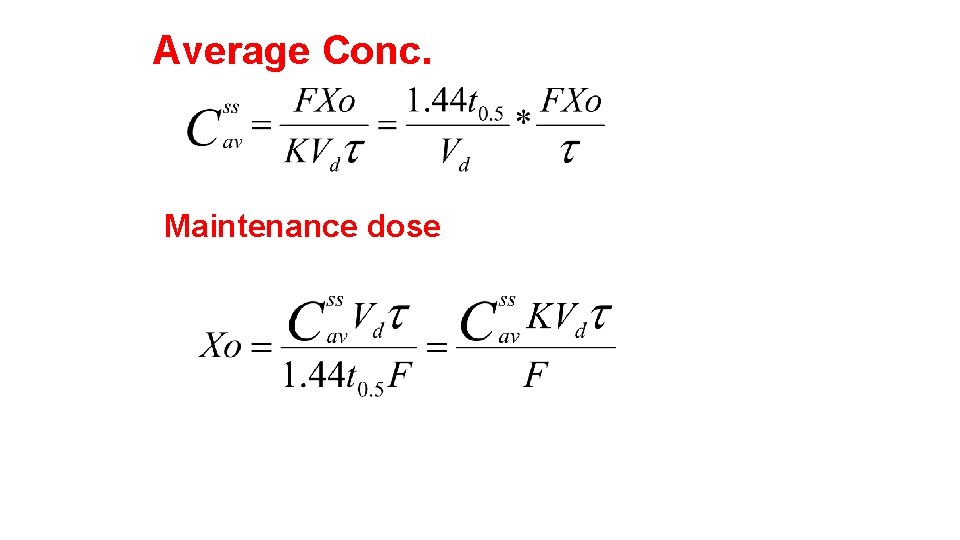
Dosage Regimen Design Based on Average Conc. At SS The Css, av or log Dose-Response or Therapeutic Window Method (see Figures 28 -1 and 28 -2) is used for those drugs whose clinical effect follows a log dose-response curve and an average concentration is desired within a therapeutic range. Drugs that are often based on this pattern are lidocaine, procainamide, theophylline, quinidine, bactericidal antibiotics, analgesics, antipyretics, and hypoglycemic agents.


Average Conc. Maintenance dose

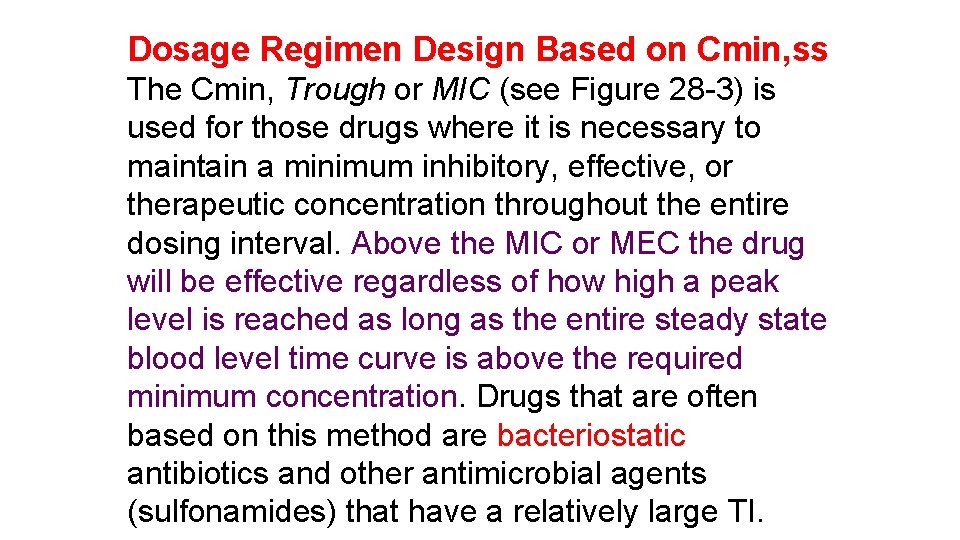
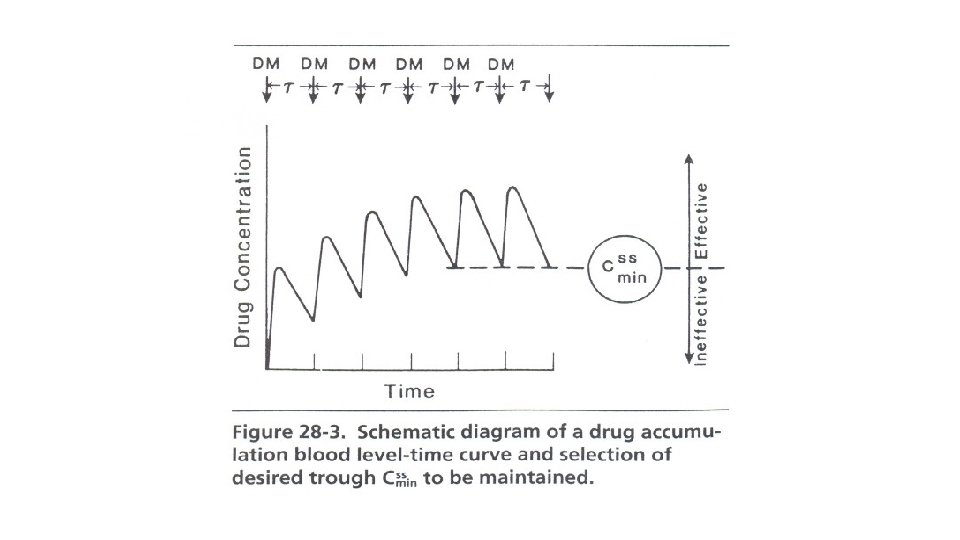
Dosage Regimen Design Based on Cmin, ss The Cmin, Trough or MIC (see Figure 28 -3) is used for those drugs where it is necessary to maintain a minimum inhibitory, effective, or therapeutic concentration throughout the entire dosing interval. Above the MIC or MEC the drug will be effective regardless of how high a peak level is reached as long as the entire steady state blood level time curve is above the required minimum concentration. Drugs that are often based on this method are bacteriostatic antibiotics and other antimicrobial agents (sulfonamides) that have a relatively large TI.

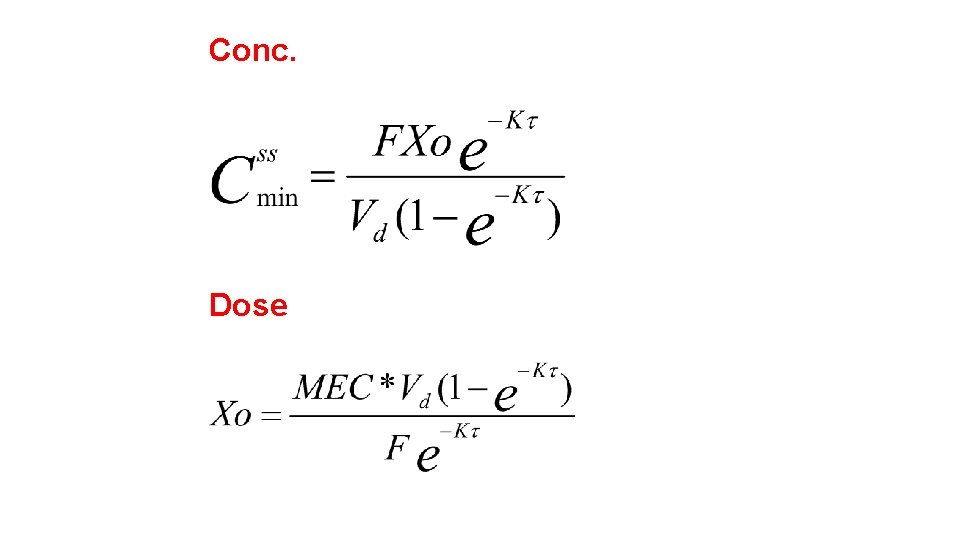
Conc. Dose

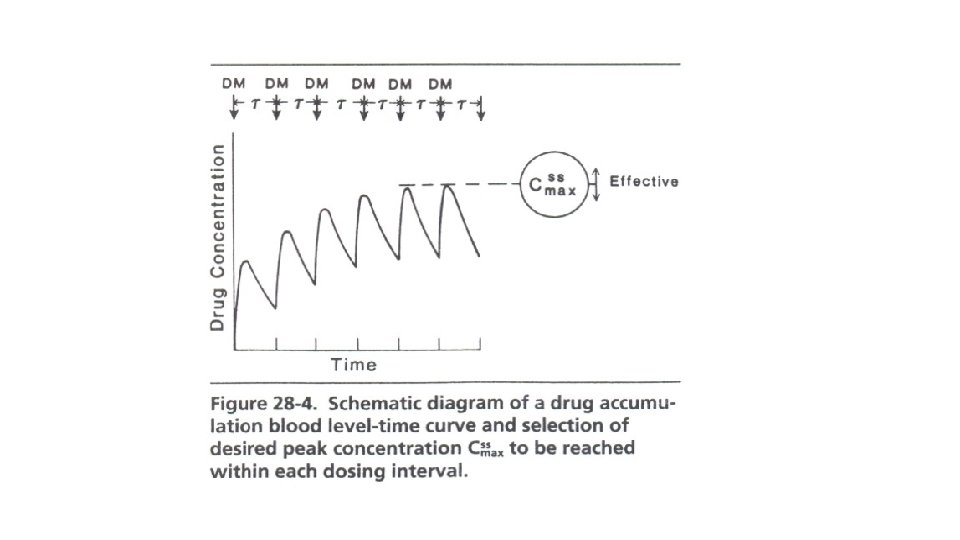
Dosage Regimen Design Based on Cmax, ss The Peak Method (see Figure 28 -4) is used for those drugs for which it is desired to reach a certain peak concentration within each dosing interval. This is particularly the case with bactericidal drugs which act only on the proliferating microorganisms. In these cases one does not wish to inhibit the growth of those microorganisms that have not been killed by the previous dose. Drugs often based on the Cmax method are penicillins, cephalosporins, gentamicin, kanamycin, etc.

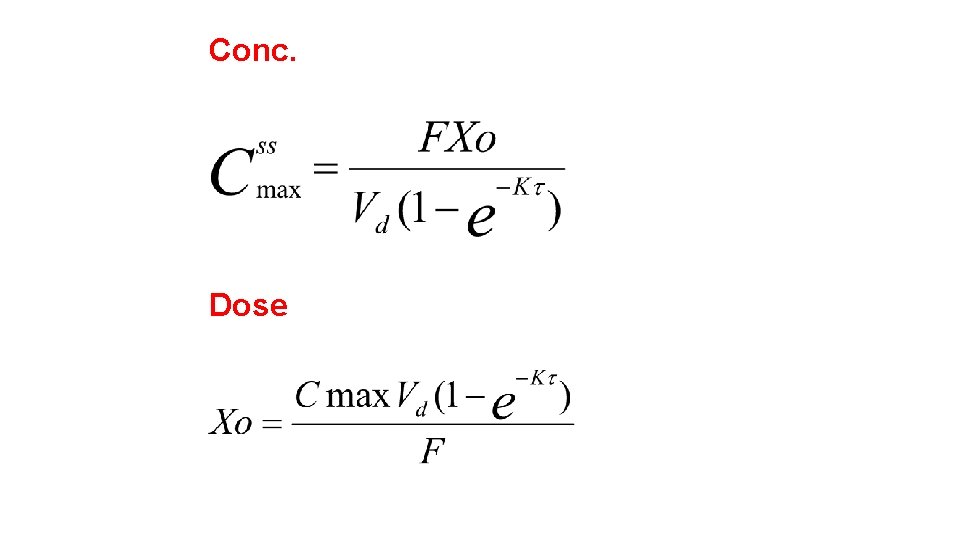
Conc. Dose

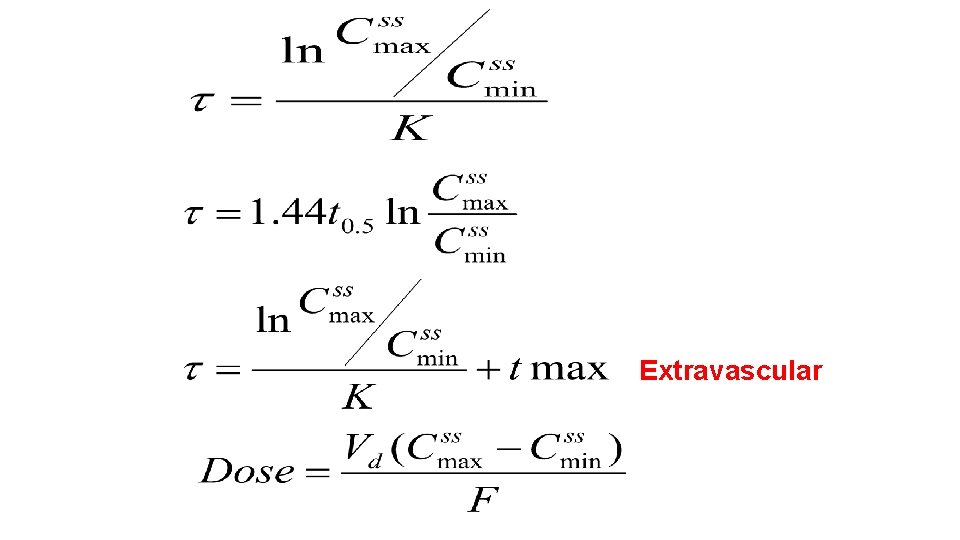
Dosage Regimen Design Based on Fixed Cmax. Cmin The fixed Cmax- Cmin or limited Fluctuation Method (see Figure 28 -5) may be used for drugs to be dosed according to a pattern where within a dosing interval the steady state blood levels shall not exceed a maximum level Css, max and not undercut a desired minimum level Css, min. Drugs often based on this method are the aminoglycosides, and theophylline. Having the range of fluctuations set, first the necessary dosing interval is calculated

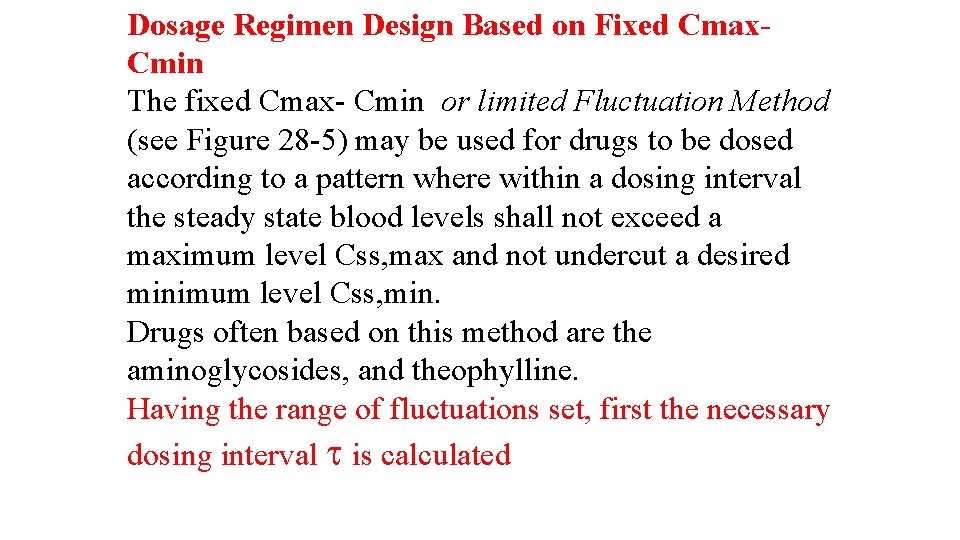
Relationship between pilocarpine plasma level and saliva flow(weaveretal. )


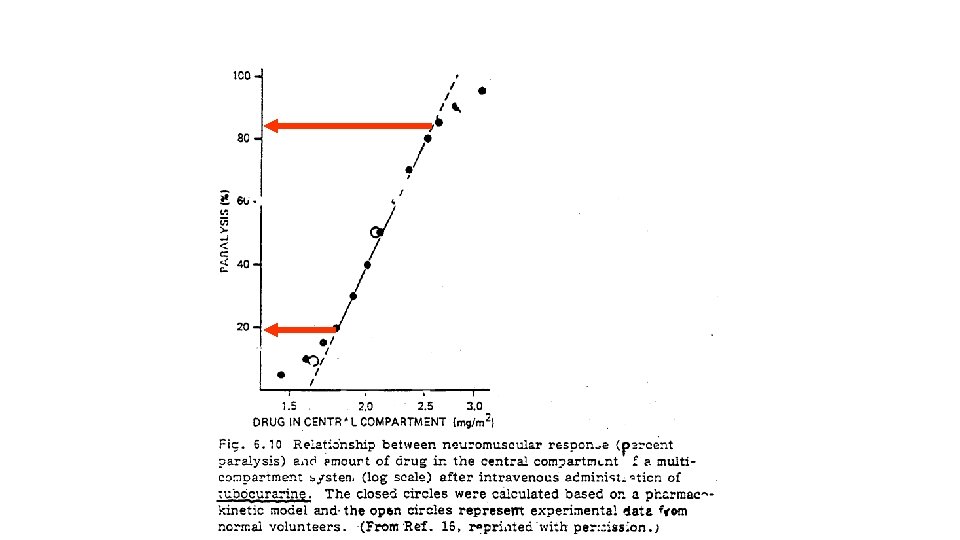
Log-Linear Model

Extravascular

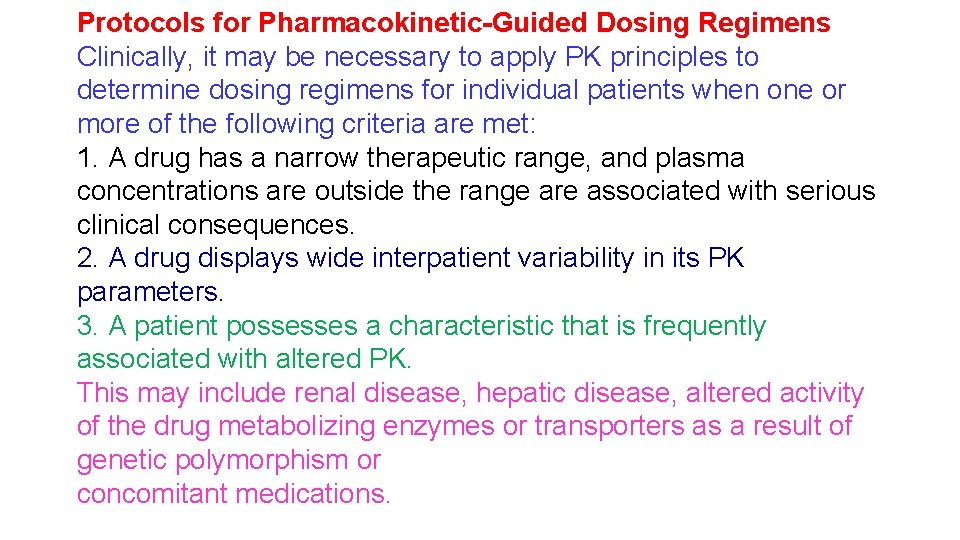
Protocols for Pharmacokinetic-Guided Dosing Regimens Clinically, it may be necessary to apply PK principles to determine dosing regimens for individual patients when one or more of the following criteria are met: 1. A drug has a narrow therapeutic range, and plasma concentrations are outside the range are associated with serious clinical consequences. 2. A drug displays wide interpatient variability in its PK parameters. 3. A patient possesses a characteristic that is frequently associated with altered PK. This may include renal disease, hepatic disease, altered activity of the drug metabolizing enzymes or transporters as a result of genetic polymorphism or concomitant medications.

Drugs that are commonly subject to pharmacokineticbased dosage individualization include aminoglycosides, phenytoin, lithium, immunosuppressants, and digoxin. Warfarin is also a very important example of a drug whose dose must be individualized for each patient. However, since the response to warfarin is easily measured (clotting time or international normalized ratio), dosage adjustments to warfarin are based on the response itself rather than on plasma concentrations and pharmacokinetics.

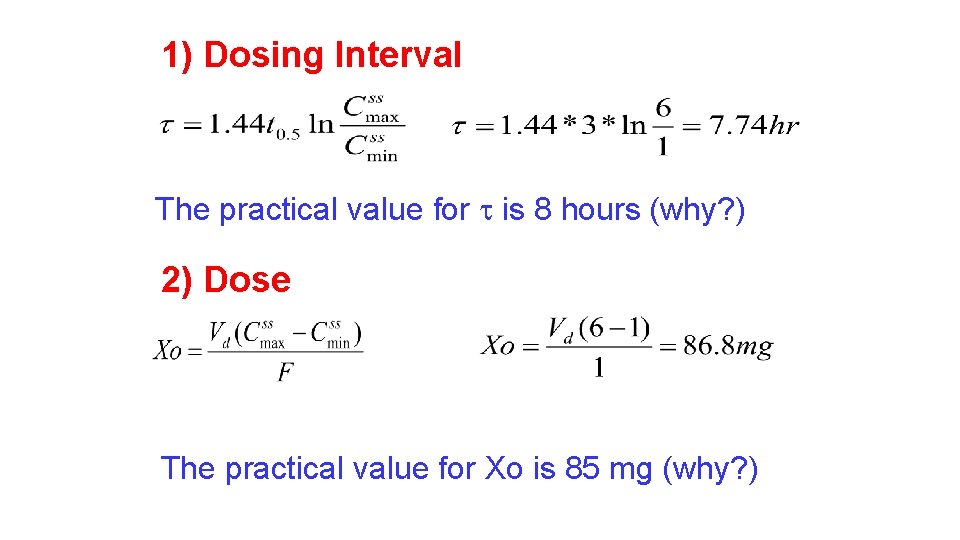
Case Study: Calculate an appropriate dosing regimen for the following male patient (Xo, , XL); age = 56 years, weight = 62 kg. Normally this drug has a half-life of 3 hours and 70% excreted in urine. Apparent volume of distribution is calculated as 0. 28 L/Kg. Develop a dosing regimen to keep the peak concentration close to but below 6 µg/ml and the trough concentration below but close to 1. 0 µg/ml.

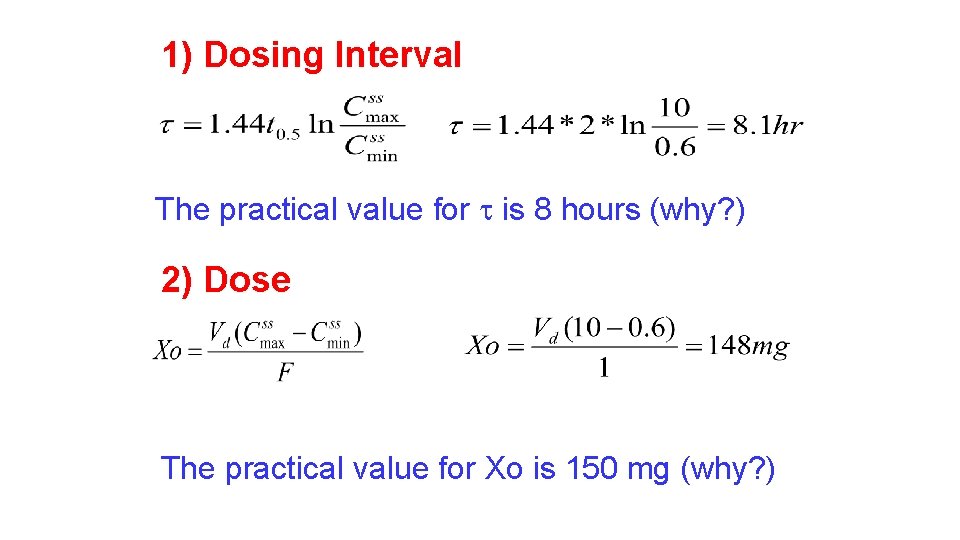
1) Dosing Interval The practical value for is 8 hours (why? ) 2) Dose The practical value for Xo is 85 mg (why? )

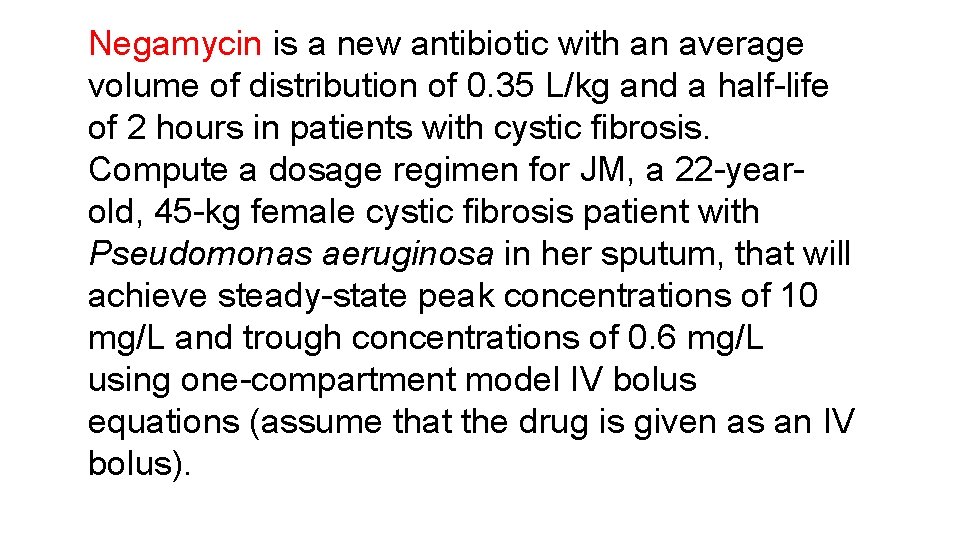
Negamycin is a new antibiotic with an average volume of distribution of 0. 35 L/kg and a half-life of 2 hours in patients with cystic fibrosis. Compute a dosage regimen for JM, a 22 -yearold, 45 -kg female cystic fibrosis patient with Pseudomonas aeruginosa in her sputum, that will achieve steady-state peak concentrations of 10 mg/L and trough concentrations of 0. 6 mg/L using one-compartment model IV bolus equations (assume that the drug is given as an IV bolus).

1) Dosing Interval The practical value for is 8 hours (why? ) 2) Dose The practical value for Xo is 150 mg (why? )

Following a severe blow to the head, an 80 -kg man (L. K. ) developed seizures. Recommend a suitable dosing regimen for phenobarbital sodium. Therapeutic range = 10– 30 mg/L, F = 0. 9, S = 0. 9, Vd = 0. 7 L/kg, and Cl = 4. 0 m. L/(h ⋅ kg). A maintenance dose of 190 mg of phenobarbital sodium daily is recommended, with a loading dose of around 1500 mg. Generally, the loading dose is divided into three or four smaller units that can be administered over a period of several hours.

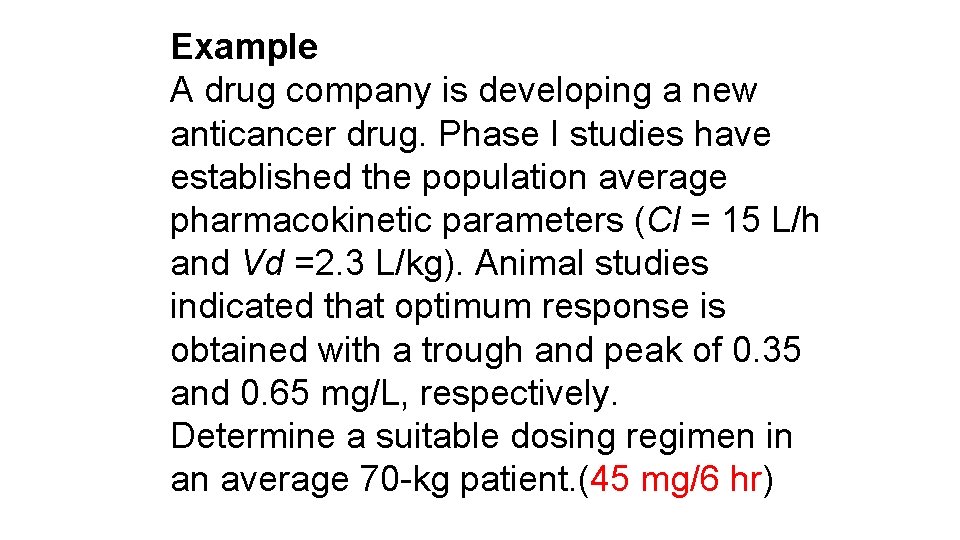
Example A drug company is developing a new anticancer drug. Phase I studies have established the population average pharmacokinetic parameters (Cl = 15 L/h and Vd =2. 3 L/kg). Animal studies indicated that optimum response is obtained with a trough and peak of 0. 35 and 0. 65 mg/L, respectively. Determine a suitable dosing regimen in an average 70 -kg patient. (45 mg/6 hr)

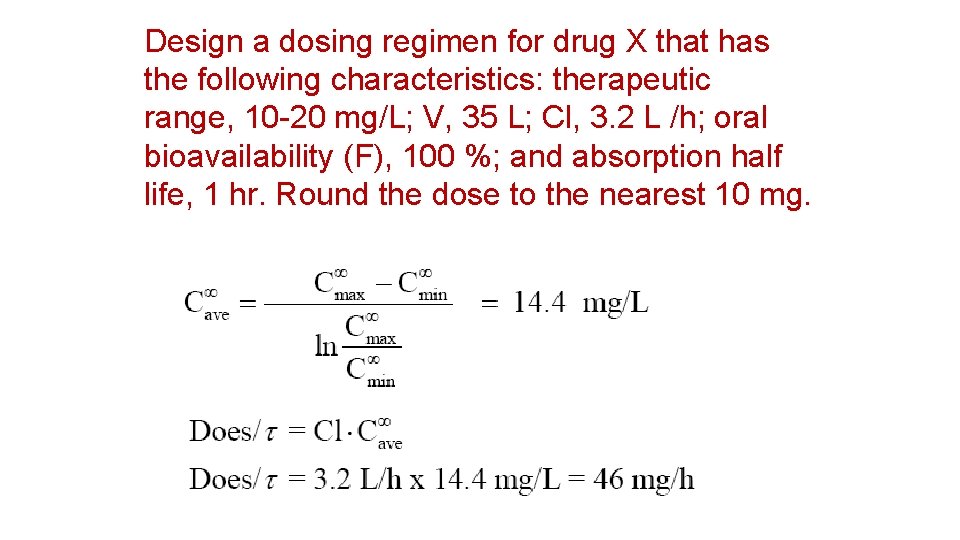
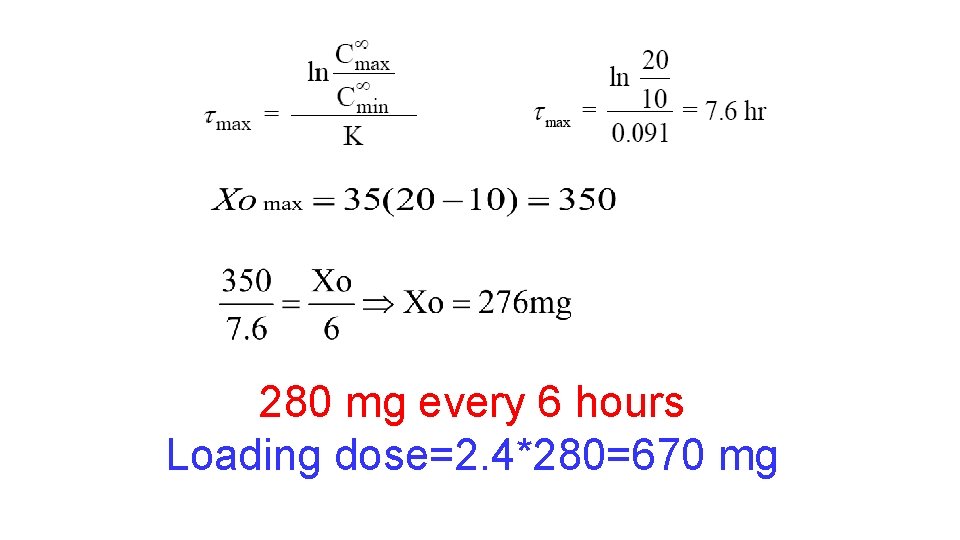
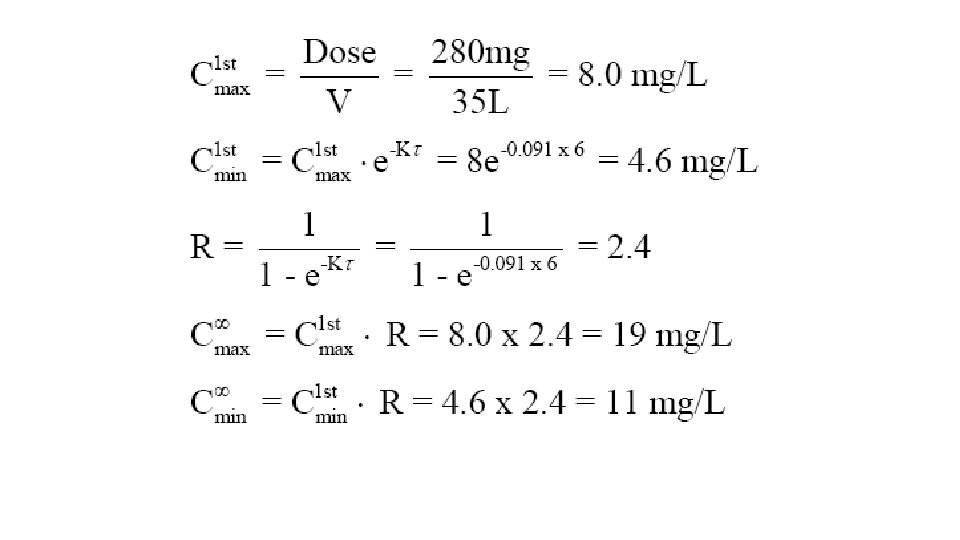
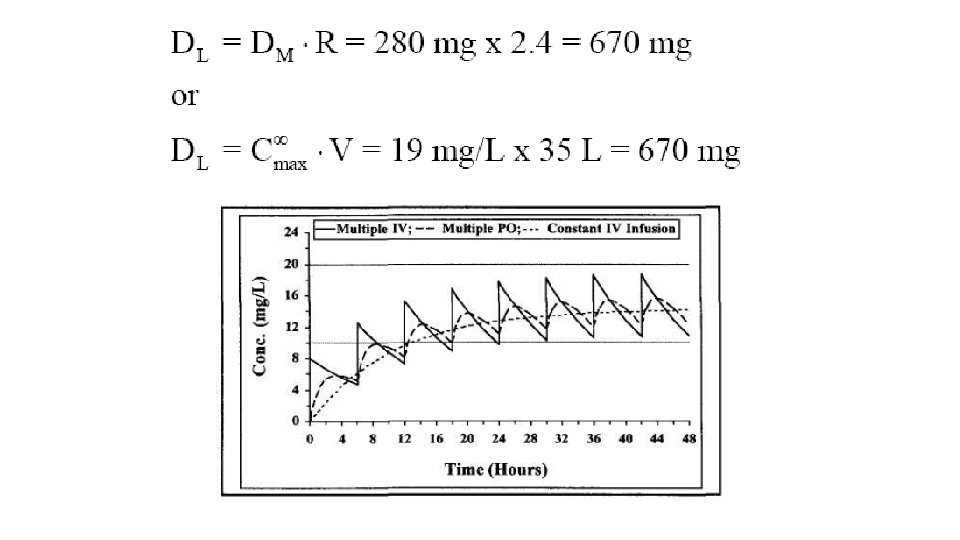
Design a dosing regimen for drug X that has the following characteristics: therapeutic range, 10 -20 mg/L; V, 35 L; Cl, 3. 2 L /h; oral bioavailability (F), 100 %; and absorption half life, 1 hr. Round the dose to the nearest 10 mg.

280 mg every 6 hours Loading dose=2. 4*280=670 mg



Case study The volume of distribution (V) and clearance (Cl) of a cardiac drug are 40 L and 2. 31 L/h, respectively. The therapeutic range of the drug is between 2 and 5 mg/L. I. Determine a dosage regimen (dose, dosage interval, and loading dose) for this drug to be administered via IV bolus method. II. What are the predicted maximum, minimum, and average plasma concentrations at steady state using your recommendation in I?

After a month of therapy, the patient receives a second drug which results in doubling the Cl of the cardiac drug. III. What are the V and t 1/2 of the drug in the presence of the interacting drug? IV. What would happen to the average, minimum, and maximum steady-state plasma concentrations of the drug? V. How would you change the dosage regimen (dose and/or dosage interval) of this drug, if any?

Case 2 0 0 • A patient is taking 1000 mg sulfamethoxazole tablet every 12 hours for the treatment of urinary tract infection. Sulfamethoxazole is rapidly and completely absorbed after oral administration. What is the amount of sulfamethoxazole eliminated during one dosing interval at steady state ? 0 1

Case 3 0 4 5. 1 8 = o C • A patient is receiving 1000 mg of sulfamethoxazole iv every 12 hours for the treatment of severe gramnegative infection. At steady state the maximum and minimum serum sulfamethoxazole concentrations were 81. 5 mg/L and 40 mg/L, respectively. What is the volume of distribution of sulfamethoxazole ?

Case 4 / C U A = • During repeated administration of 60 mg indomethacin tablet every 12 hours (F=1) for the treatment of rheumatoid arthritis the AUC during one dosing interval was 8 mg-hr/L. What is the average indomethacin steady state plasma concentration during this dosing regimen (60 mg every 12 hours) ? v a C

BJ is a 62 -year-old, 70 -kg female with a ventricular arrhythmia. Assuming a V = 33 L and Cl = 0. 5 L/min, use onecompartment model equations to compute a lidocaine IV bolus loading dose (to be administered over 1– 2 minutes) and continuous infusion to achieve a Css = 3 mg/L.

MM is a 54 -year-old, 68 -kg male being treated with procainamide 750 -mg regular release capsules every 6 hours for an arrhythmia. The following steadystate concentration is available: Cssmin = 1. 5 mg/L (obtained immediately predose). Calculate a dose that will achieve a Cssmin = 2. 5 mg/L. 2 1 0 5

JB is a 78 -year-old, 100 -kg male being treated with digoxin for heart failure. While receiving digoxin tablets 125 μg daily, a steady-state digoxin concentration equal to 0. 6 μg/L is obtained. (A) Assuming F = 0. 7, compute digoxin clearance for the patient using the average steady-state concentration equation. (B) Compute a new digoxin tablet dose for the patient that will achieve Css = 1. 2 μg/L.

QJ is a 67 -year-old, 80 -kg male being treated for chronic obstructive pulmonary disease. Sustained-release oral theophylline is being added to his drug regimen. Assuming F = 1. 0, V = 40 L, and t 1/2 = 5 hours, compute an oral theophylline dose to be administered every 12 hours that would achieve a Css = 8 mg/L using the average steady-state concentration equation.
 Loading dose formula
Loading dose formula Accumulation index pharmacokinetics
Accumulation index pharmacokinetics When will i use polynomials in real life
When will i use polynomials in real life Other practical applications of boyle's law
Other practical applications of boyle's law Practical application of biotechnology
Practical application of biotechnology One empire one god one emperor
One empire one god one emperor One one one little puppy run
One one one little puppy run One king one law one faith
One king one law one faith One god one empire one emperor
One god one empire one emperor One ford
One ford See one do one teach one
See one do one teach one One price policy
One price policy Willow cabin speech
Willow cabin speech Studiendekanat uni bonn
Studiendekanat uni bonn Asean tourism strategic plan
Asean tourism strategic plan Asean one vision one identity one community
Asean one vision one identity one community Tigasone
Tigasone Regimen integrado
Regimen integrado Que es regimen general
Que es regimen general Oraciones subordinadas sustantivas enunciativas
Oraciones subordinadas sustantivas enunciativas Complemento de regimen preposicional
Complemento de regimen preposicional Comercio en el antiguo regimen
Comercio en el antiguo regimen Emaco regimen
Emaco regimen Regimen pro pyme general (14d) 3
Regimen pro pyme general (14d) 3 Rockey davis incision
Rockey davis incision Ochsner sherren regime
Ochsner sherren regime Características del modelo tradicional de la educación
Características del modelo tradicional de la educación Reforma pro salud
Reforma pro salud Emaco regimen
Emaco regimen Dyspermy
Dyspermy Programa de reintegro laboral arl sura
Programa de reintegro laboral arl sura Ageloc body shaping gel regimen
Ageloc body shaping gel regimen Regimen populista caracteristicas
Regimen populista caracteristicas Regimen sancionatorio ambiental
Regimen sancionatorio ambiental Régimen disciplinario losep
Régimen disciplinario losep Drug class
Drug class Regimen complementario al impuesto al valor agregado
Regimen complementario al impuesto al valor agregado Complemento de regimen
Complemento de regimen Regimen exportacion
Regimen exportacion Complement de regim
Complement de regim Clases de oración compuesta
Clases de oración compuesta El antiguo regimen
El antiguo regimen Pid opd regimen
Pid opd regimen Zuspan regimen
Zuspan regimen Antiguo regimen definicion
Antiguo regimen definicion Regimen hibrido
Regimen hibrido Regimen patrimonial del estado
Regimen patrimonial del estado One stanza from poetry for practical criticism
One stanza from poetry for practical criticism Eurocode 2 lap length table
Eurocode 2 lap length table Types of costume design
Types of costume design Performance based practical design
Performance based practical design