Done by Ahmed Fahad Basilim clinical pharmacist intern

Done by : Ahmed Fahad Basilim clinical pharmacist intern Supervised by: Dr. Muna Filfilan
™ Nplate - Romiplostim

Nplate™ - Romiplostim - Manufacturer: Amgen, Inc. - FDA Approval Date: 08/2008

Nplate™ - Romiplostim - What is Nplate?
Nplate™ - Romiplostim - What is Nplate? Nplate is a man-made protein medicine used to treat low blood platelet counts in adults. Romiplostim is produced by recombinant DNA technology in Escherichia coli (E coli). - Mechanism of Action:
Nplate™ - Romiplostim - What is Nplate? Nplate is a man-made protein medicine used to treat low blood platelet counts in adults. Romiplostim is produced by recombinant DNA technology in Escherichia coli (E coli). Mechanism of Action: Is an antihemorrhagic. This member of the thrombopoietin mimetic class is an Fc-peptide fusion protein (peptibody) that activates intracellular transcriptional pathways leading to increased platelet production via the thrombopoietin receptor.
Nplate™ - Romiplostim - Indication: Thrombocytopenia in patients with chronic immune thrombocytopenic purpura who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Nplate™ - Romiplostim - Indication: Thrombocytopenia in patients with chronic immune thrombocytopenic purpura who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. - Place in therapy: Treat ITP in patients with PLT <50, 000 despite above therapies.
Nplate™ - Romiplostim - Indication: Thrombocytopenia in patients with chronic immune thrombocytopenic purpura who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. - Place in therapy: Treat ITP in patients with PLT <50, 000 despite above therapies. - Nplate should not be used in an attempt to normalize platelet counts.
Nplate™ - Romiplostim - Pharmacokinetics: - Peak: 7 to 50 hr (median 14 hr) - t 1/2 : 1 -34 days (median 3. 5 days)
Nplate™ - Romiplostim - Dosing: -Initial: 1 mcg/kg SQ weekly based on TBW - Increase by 1 mcg/kg until PLT > 50, 000. - If PLT > 200, 000 for 2 weeks, ↓ by 1 mcg/kg. - If PLT > 400, 000, hold. After PLT < 200, 000 resume at dose ↓ by 1 mcg/kg. - Average dose: 2 mcg/kg/wk - Max dose: 10 mcg/kg/wk
Nplate™ - Romiplostim - Discontinuation: - Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding. - After 4 weeks of Nplate therapy at the maximum weekly dose of 10 mcg/kg , Obtain CBCs, including platelet counts, weekly for at least 2 weeks following discontinuation of Nplate.
Nplate™ - Romiplostim -Efficacy Monitoring: - Monitor CBCs, including platelet counts and peripheral blood smears, weekly until a stable Nplate dose has been achieved. - Thereafter, monitor CBCs, including platelet counts and peripheral blood smears, at least monthly.
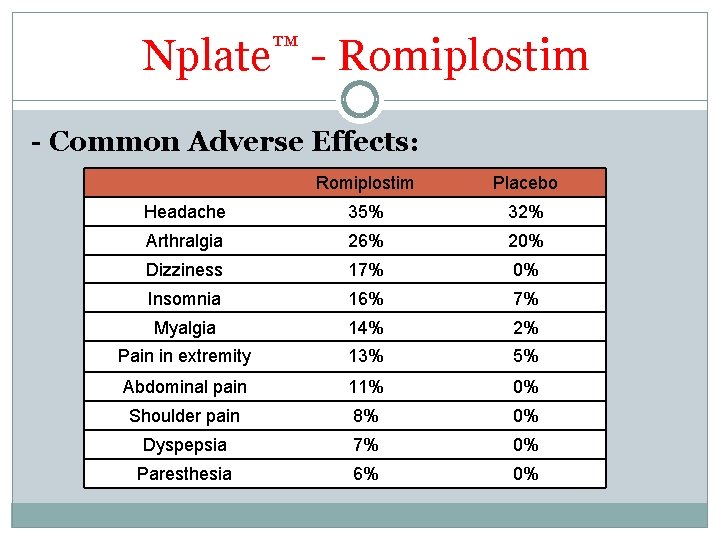
Nplate™ - Romiplostim - Common Adverse Effects: Romiplostim Placebo Headache 35% 32% Arthralgia 26% 20% Dizziness 17% 0% Insomnia 16% 7% Myalgia 14% 2% Pain in extremity 13% 5% Abdominal pain 11% 0% Shoulder pain 8% 0% Dyspepsia 7% 0% Paresthesia 6% 0%
Nplate™ - Romiplostim - Immunogenicity: As with all therapeutic proteins, patients may develop antibodies to therapeutic protein. Patients were screened for immunogenicity to romiplostim using a BIAcore-based biosensor immunoassay. This assay is capable of detecting both high- and low-affinity binding antibodies that bind to romiplostim and cross-react with TPO.
Nplate™ - Romiplostim - Serious Adverse Effects: -Bone marrow reticulin deposition - Romiplostim: 4%, Placebo 0% - Worsening thrombocytopenia after discontinuation - Romiplostim: 7%, Placebo 0%
Nplate™ - Romiplostim - Contraindications: - None listed - Warnings: - Risk for bone marrow fibrosis - Hematologic malignancies - Thromboembolism - Lack of response: determine cause - Precautions: - Hepatic impairment - Renal impairment - Pediatrics

Nplate™ - Romiplostim -Drug Interactions: - No formal studies conducted.

Nplate™ - Romiplostim - Drug Facts: - Romiplostim is a novel thrombopoietin peptide mimetic which increases platelet production by binding to and activating the thrombopoietin receptor. - The platelet count will increase in 4 -9 days and peaks in 12 -16 days.

Nplate™ - Romiplostim - Summary: - There are limited FDA approved treatments (i. e. , corticosteroids, immunglobulins) or surgical therapy (removal of the spleen) for adult patients with chronic ITP.
Nplate™ - Romiplostim - Summary: - There are limited FDA approved treatments (i. e. , corticosteroids, immunglobulins) or surgical therapy (removal of the spleen) for adult patients with chronic ITP. - Nplate is first and only available agent which treats ITP by increasing platelet production through activation of the thrombopoietin receptor.
Nplate™ - Romiplostim - Summary: - Romiplostim is not intended to normalize platelet counts. It should be titrated weekly by 1 mcg/kg to achieve platelet count ≥ 50, 000 as necessary to reduce bleeding risk. Monitoring CBC, platelet counts, and peripheral blood smear is essential.
Nplate™ - Romiplostim - Summary: - Romiplostim is not intended to normalize platelet counts. It should be titrated weekly by 1 mcg/kg to achieve platelet count ≥ 50, 000 as necessary to reduce bleeding risk. Monitoring CBC, platelet counts, and peripheral blood smear is essential. - May cause rare but serious adverse effects: bone marrow reticulin formation, thromboembolism, hematologic malignancies or worsened platelet counts after cessation of therapy.

Nplate™ - Romiplostim -References: - http: //www. nplate. com
- Slides: 24