DO NOW You must be silent and Based
DO NOW � You must be silent and �Based on your quiz, what working for credit � Be on time � Enter silently � Grab papers � Immediately start work � No talking do you still need to work on? �Is oil soluble in water? �Is salt soluble in water? �What do you think “soluble” means?

ANNOUNCEMENTS �ACT – Tomorrow! �Tutoring – Today! �Students of the week! – Cynthia Crawford and Danaya Geter!

LET’S DIVE IN �Notes expectations: Silent Writing Raise hand to ask question No bathroom during this time

SATURATION �Solubility: the ability of one substance to dissolve in another at a given temp and pressure (amount of solute that will dissolve)

SATURATION �Miscible: liquids that are completely soluble in each other �Immiscible: liquids that are insoluble in each other

SATURATION � Saturated solution: a solution that cannot dissolve any more solute under the given conditions � Unsaturated solutions: contains less solute than a saturated solution, able to dissolve additional solute � Supersaturated solution: has more solute dissolved than the solubility indicates could normally be possible, however there is no excess
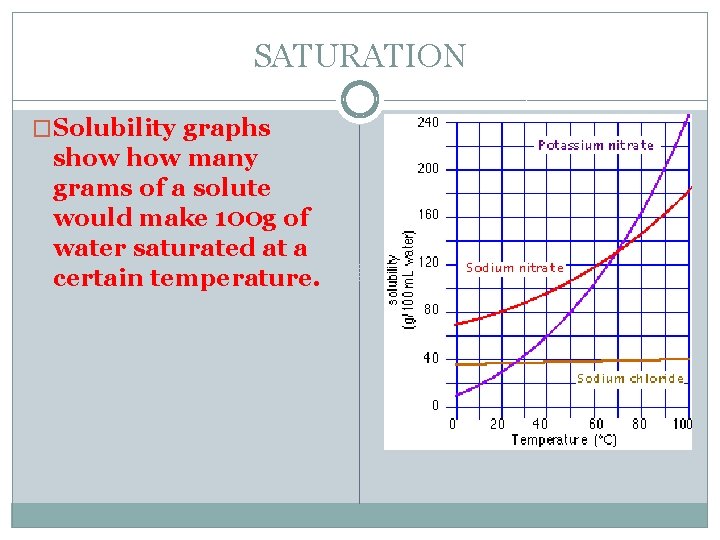
SATURATION �Solubility graphs show many grams of a solute would make 100 g of water saturated at a certain temperature.
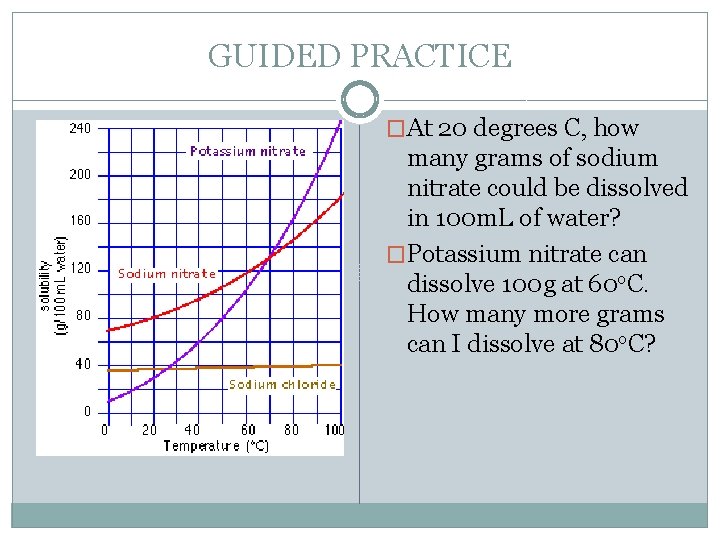
GUIDED PRACTICE �At 20 degrees C, how many grams of sodium nitrate could be dissolved in 100 m. L of water? �Potassium nitrate can dissolve 100 g at 60 o. C. How many more grams can I dissolve at 80 o. C?

INDEPENDENT PRACTICE �Worksheet �Expectations: Whisper Work with only 1 other person
- Slides: 11