DO NOW Write the formula for calcium nitride

















- Slides: 23

DO NOW

• Write the formula for calcium nitride It is Is there a metal and/or a poly? How do we know it is not a poly? Ca+2 N-3 ionic so write the charges and criss cross. Ca 3 N 2 2

• Write the formula for Iron(III) sulfate Is there a metal and/or a poly? +3 Fe (SO 4 -2 ) It is ionic so write the charges and criss cross. Fe 2(SO 4)3 3

• Write the formula for ammonium ox ide Is it ionic? It is ionic so write the charges and criss cross. Is ammonium an element or a poly? (NH 4)+1 O-2 (NH 4)2 O 4

Write the name for…. . • Is there a metal and/or a poly? Na 2 S Is the metal a transition metal? Sodium sulfide 5

Write the name for…. . • Is there a metal and/or a poly? NH 4 NO 3 2 polyatomic ions Ammonium nitrate 6

Write the name for…. . • Is there a metal and/or a poly? (NH 4)3 N 1 polyatomic ion Ammonium nitride It is ionic so we don’t worry about naming subscripts 7

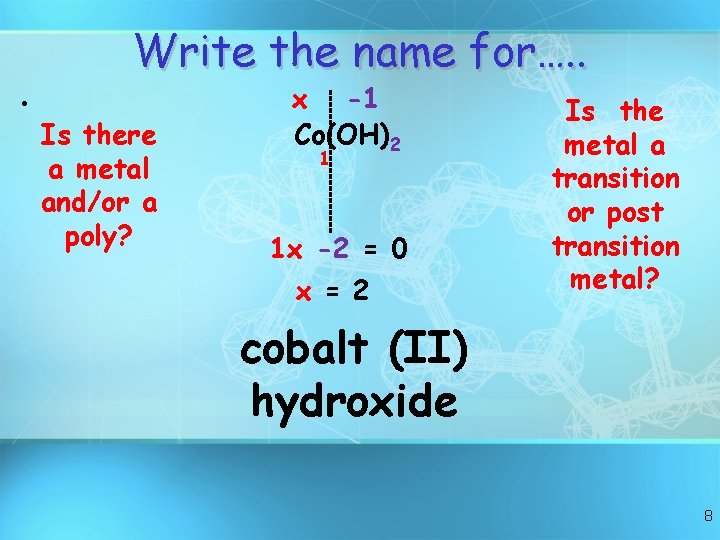
Write the name for…. . • Is there a metal and/or a poly? x -1 Co(OH)2 1 1 x -2 = 0 x = 2 Is the metal a transition or post transition metal? cobalt (II) hydroxide 8

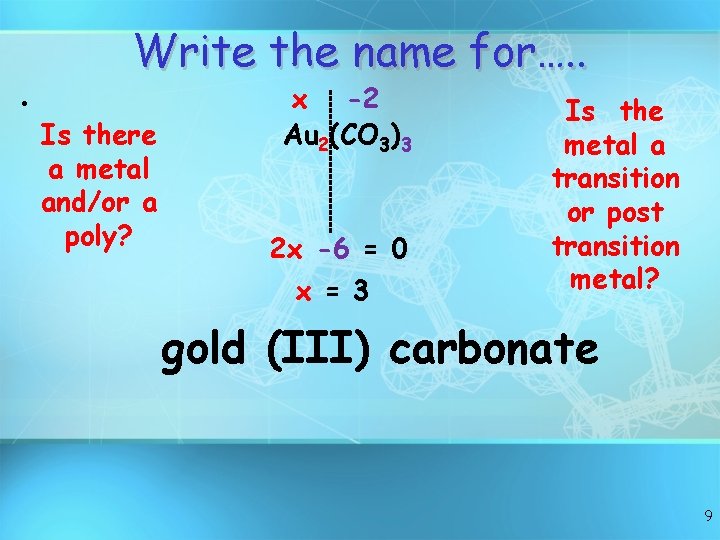
Write the name for…. . • Is there a metal and/or a poly? x -2 Au 2(CO 3)3 2 x -6 = 0 x = 3 Is the metal a transition or post transition metal? gold (III) carbonate 9

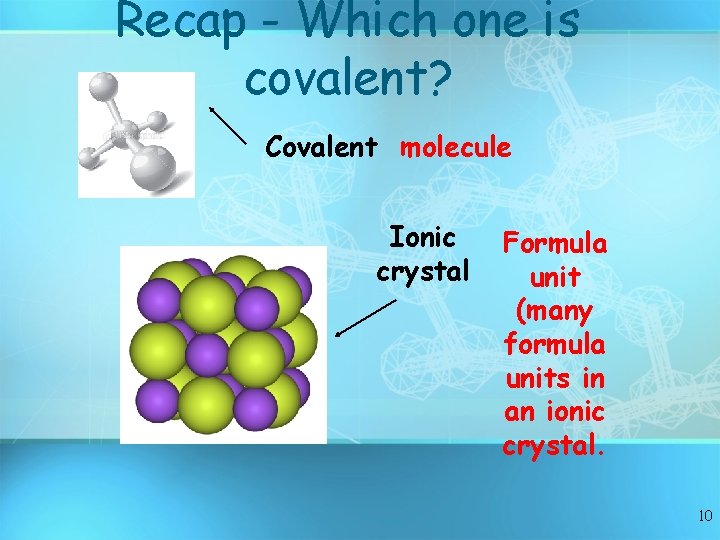
Recap - Which one is covalent? Covalent molecule Ionic crystal Formula unit (many formula units in an ionic crystal. 10

• When we write the formula for an ionic compound, do we care about the subscripts? For instance, Al. F 3 is ionic. Name this compound. a)Aluminum fluoride b)Aluminum trifluoride? 11

• When we name covalent compounds (molecular), We do care about the subscripts? YES, remember the man who inhaled SF 6 How was that named in the video? Sulfur hexafluoride 12

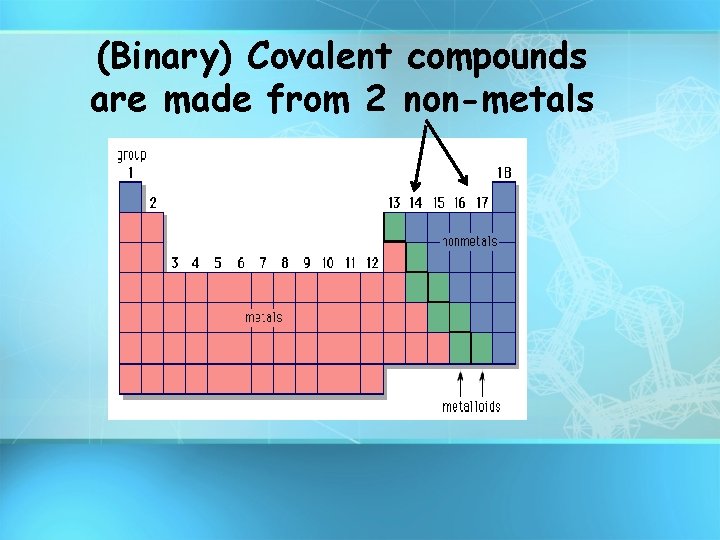
(Binary) Covalent compounds are made from 2 non-metals

• For Covalent Compounds, All subscripts are named with prefixes N 2 O 5 dinitrogen pentoxide 14

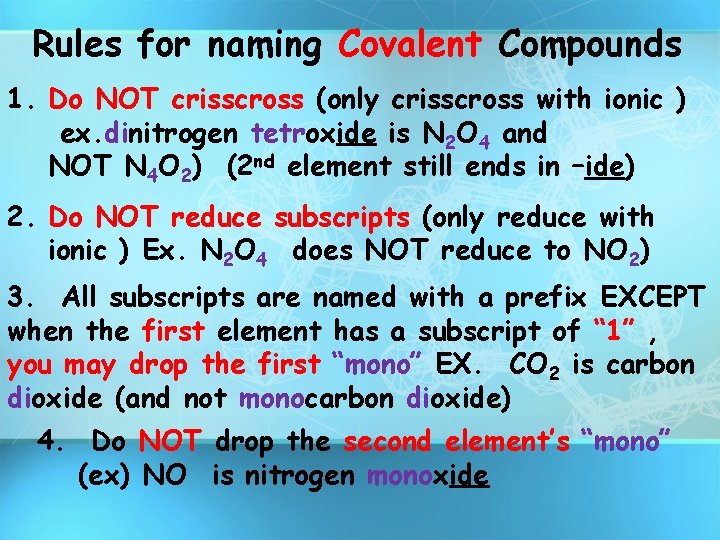
Rules for naming Covalent Compounds 1. Do NOT crisscross (only crisscross with ionic ) ex. dinitrogen tetroxide is N 2 O 4 and NOT N 4 O 2) (2 nd element still ends in –ide) 2. Do NOT reduce subscripts (only reduce with ionic ) Ex. N 2 O 4 does NOT reduce to NO 2) 3. All subscripts are named with a prefix EXCEPT when the first element has a subscript of “ 1” , you may drop the first “mono” EX. CO 2 is carbon dioxide (and not monocarbon dioxide) 4. Do NOT drop the second element’s “mono” (ex) NO is nitrogen monoxide

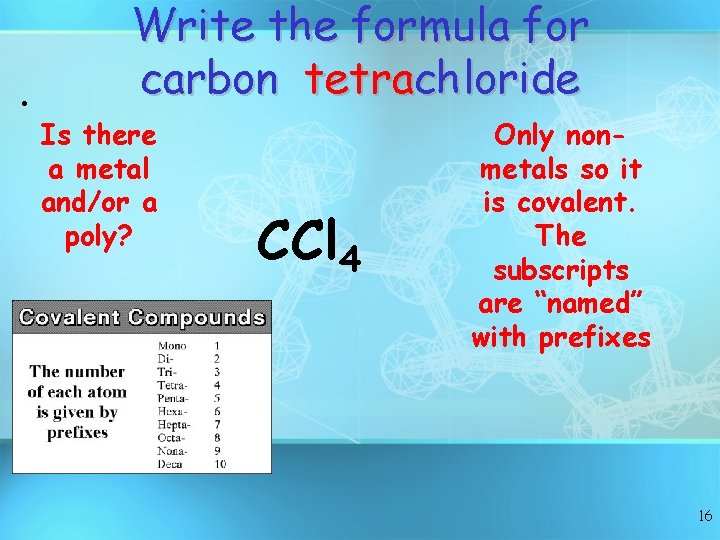
• Write the formula for carbon tetrachloride Is there a metal and/or a poly? CCl 4 Only nonmetals so it is covalent. The subscripts are “named” with prefixes 16

• Write the name for Si 2 Br 6 disilicon hexabromide 17

• Write the formula for boron triiodide BI 3 18

• Write the name for P 4 S 5 Tetraphosphorus pentasulfide 19

• Write the name for K 2 S Is it dipotassium monosulfide? NO, this is ionic so no prefixes! (No mono, di, tri…. ) potassium sulfide 20

Work in your groups #1 -7 on the worksheet.

Write the name for…. . • Is there a metal and/or a poly? x -2 Cr 2(SO 4)3 2 x -6 = 0 x = 3 Is the metal a transition or post transition metal? chromium (III) sulfate 22

• Write the formula for Iron(II) hydroxide Is there a metal and/or a poly? +2 -1 Fe (OH) It is ionic so write the charges and criss cross. Is hydroxide an element or a poly? Fe(OH)2 23