DO NOW Which liquid has the highest density







- Slides: 19

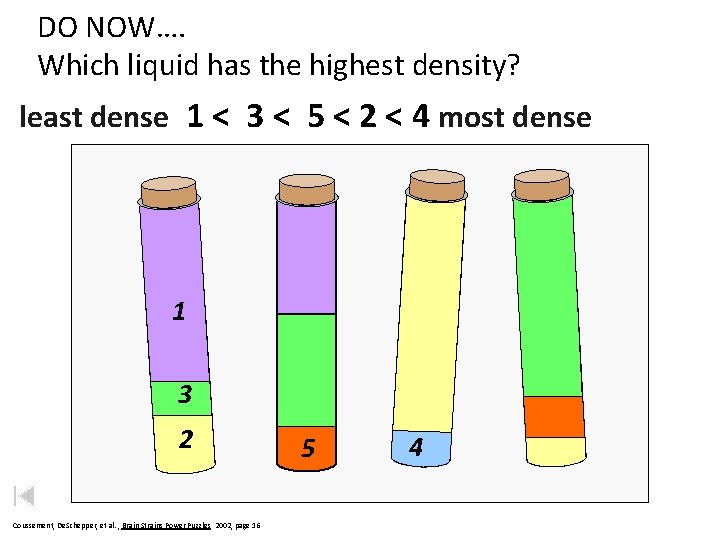
DO NOW…. Which liquid has the highest density? least dense 1 < 3 < 5 < 2 < 4 most dense 1 3 2 Coussement, De. Schepper, et al. , Brain Strains Power Puzzles 2002, page 16 5 4

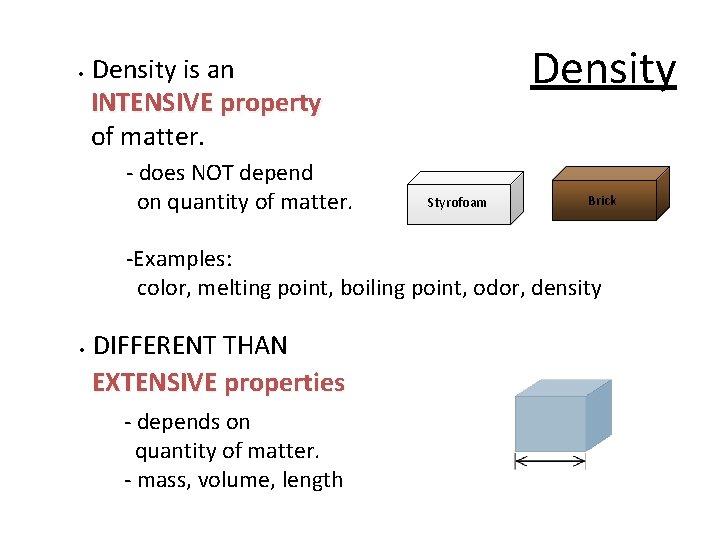
• Density is an INTENSIVE property of matter. - does NOT depend on quantity of matter. Styrofoam Brick -Examples: color, melting point, boiling point, odor, density • DIFFERENT THAN EXTENSIVE properties - depends on quantity of matter. - mass, volume, length

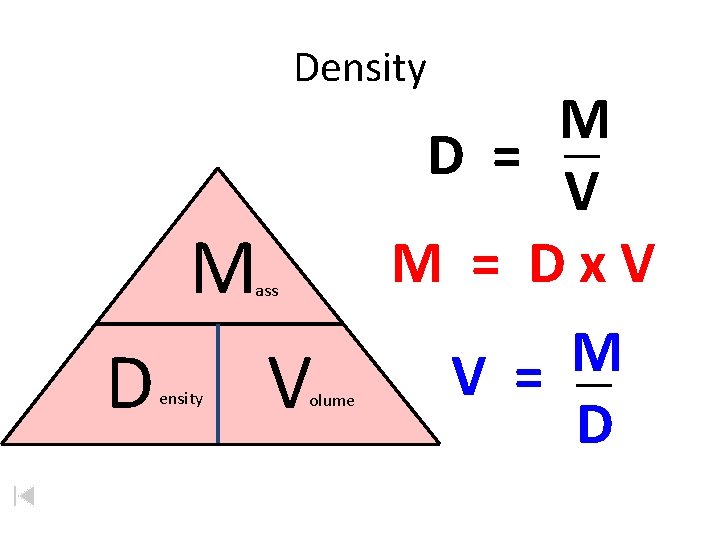
Density M ass D ensity V olume M D = V M = Dx. V M V = D

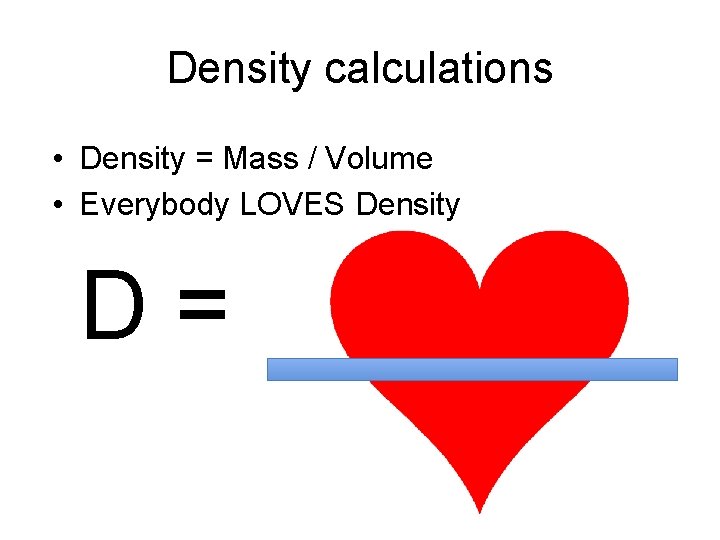
Density calculations • Density = Mass / Volume • Everybody LOVES Density D=

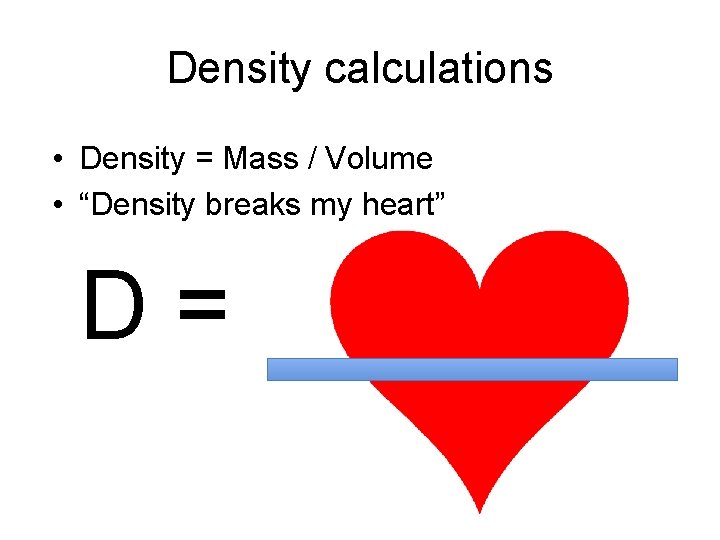
Density calculations • Density = Mass / Volume • “Density breaks my heart” D=

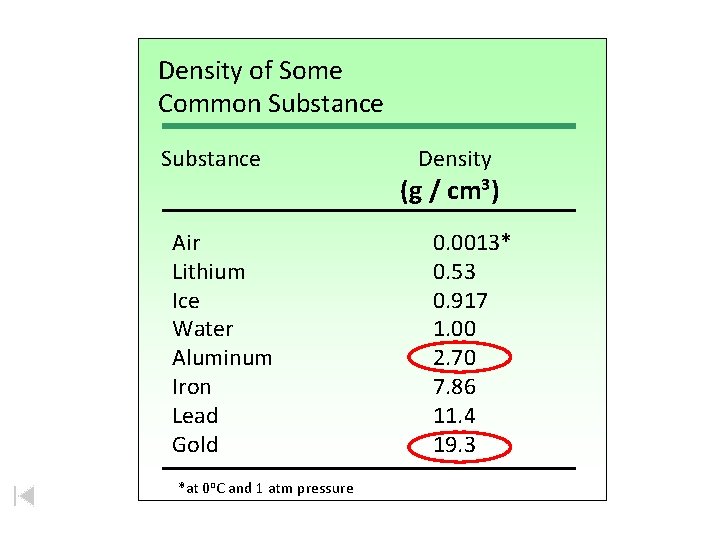
Density of Some Common Substances Air Lithium Ice Water Aluminum Iron Lead Gold *at 0 o. C and 1 atm pressure Density (g / cm 3) 0. 0013* 0. 53 0. 917 1. 00 2. 70 7. 86 11. 4 19. 3

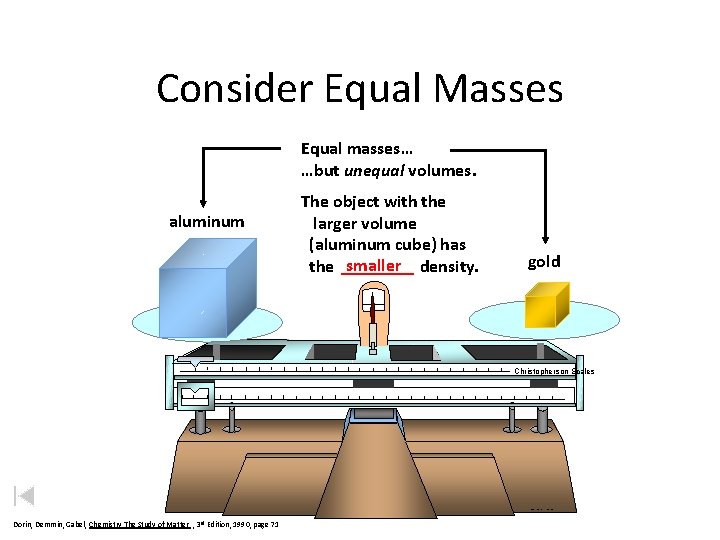
Consider Equal Masses Equal masses… …but unequal volumes. aluminum The object with the larger volume (aluminum cube) has the smaller density. gold Christopherson Scales Made in Normal, Illinois USA Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71

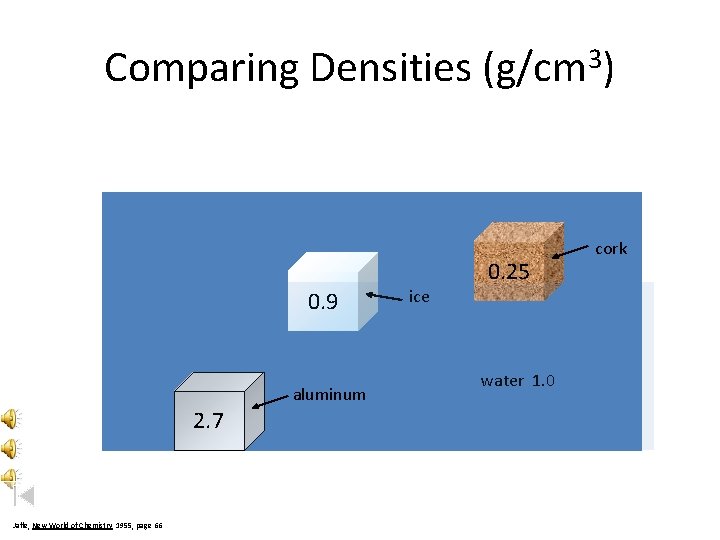
Comparing Densities (g/cm 3) 0. 25 0. 9 aluminum 2. 7 Jaffe, New World of Chemistry, 1955, page 66 ice water 1. 0 cork

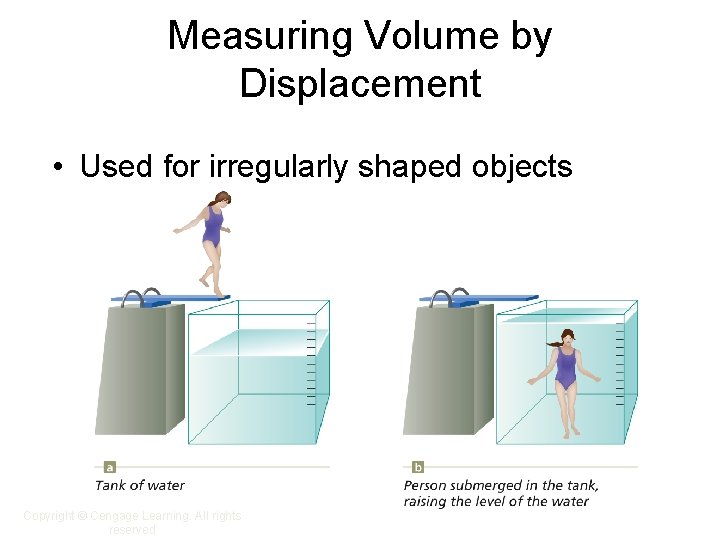
Measuring Volume by Displacement • Used for irregularly shaped objects Copyright © Cengage Learning. All rights reserved

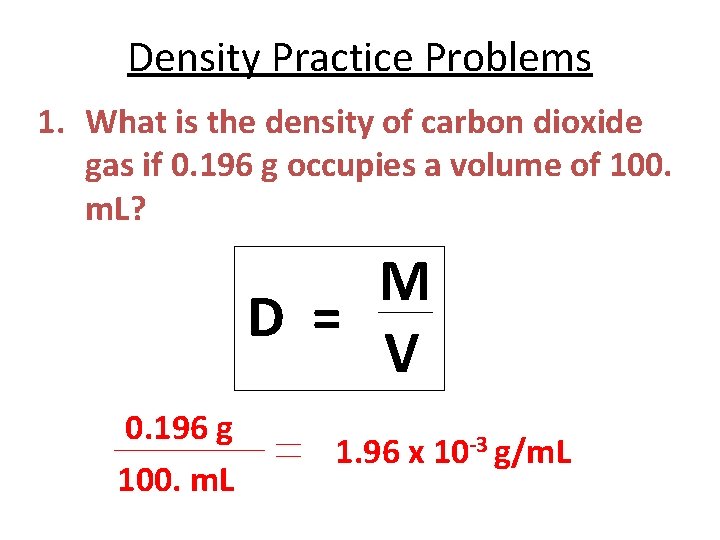
Density Practice Problems 1. What is the density of carbon dioxide gas if 0. 196 g occupies a volume of 100. m. L? M D = V 0. 196 g 100. m. L 1. 96 x 10 -3 g/m. L

Density Practice Problems 2. An irregularly shaped stone has a volume of 5. 0 m. L. The density of the stone is 1. 75 g/m. L. What is the mass of this stone? M = Dx. V 1. 75 g/m. L x 5. 0 m. L 8. 8 g

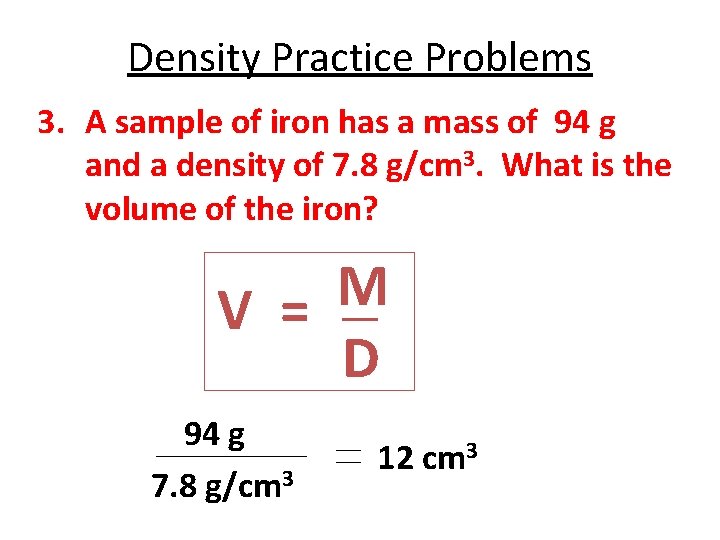
Density Practice Problems 3. A sample of iron has a mass of 94 g and a density of 7. 8 g/cm 3. What is the volume of the iron? M V = D 94 g 7. 8 g/cm 3 12 cm 3

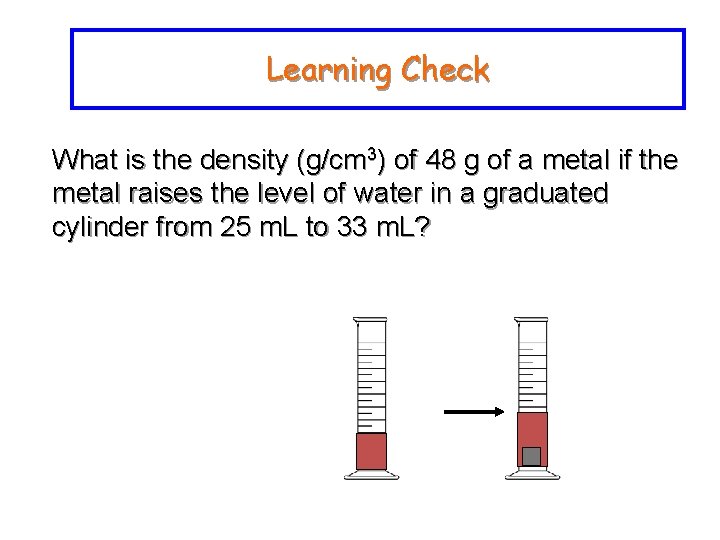
Learning Check What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L?

Look over HW: Any Q’s from HW?

Solve each of the following problems. Show the correct set-up and always use units. 1. Determine the number of mm in 1600 m. 2. Convert 2875 d. L to liters. 3. Convert 2500 m. L of hydrochloric acid (HCl) to L of HCl. 4. Convert 0. 049 kg sulfur (S) to g of S 5. Convert 150 mg of acetylsalicylic acid (aspirin) to g of aspirin.

1. Convert 5. 2 x 10 -4 Mm of magnesium (Mg) ribbon to mm of Mg ribbon 2. Convert 0. 020 kg of tin (Sn) to mg of Sn 3. Convert 26. 7 cm of copper (Cu) wire into dm of Cu wire. 4. Convert 0. 50 g of sodium (Na) to nanograms of Na

Gold's density is 19. 32 g/m. L convert to cg/L What is the speed of train travelling at 40 km/hr in Mm/s? Convert 3, 795 cm 3 to liters.

Converting more than one unit… Convert 1. 96 x 10 -3 g/m. L to Kg/d. L Convert 1. 96 x 10 -3 g/m. L to g/m 3
