Do Now What does a chloroplast look like
































- Slides: 75

Do Now What does a chloroplast look like? How do plants obtain energy? What is the formula for glucose? How do autotrophs obtain energy? How do heterotrophs obtain energy?

Chapter 6 Photosynthesis: Capturing and Converting Energy

Energy – the ability to do work

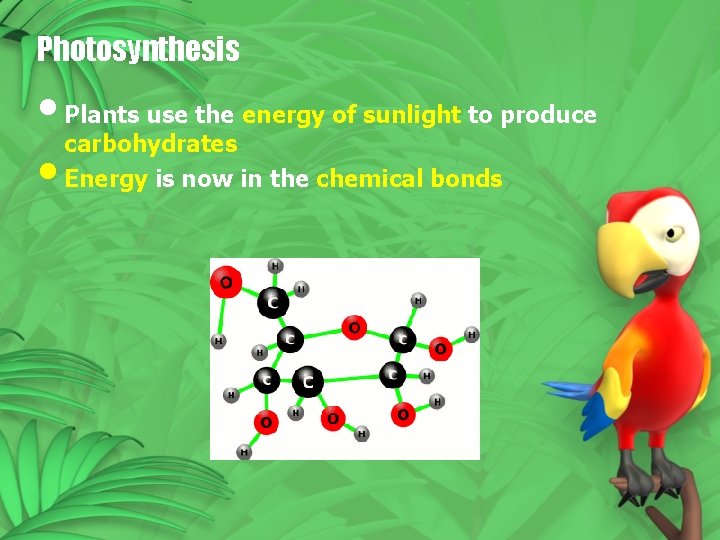
Photosynthesis • Plants use the energy of sunlight to produce carbohydrates • Energy is now in the chemical bonds

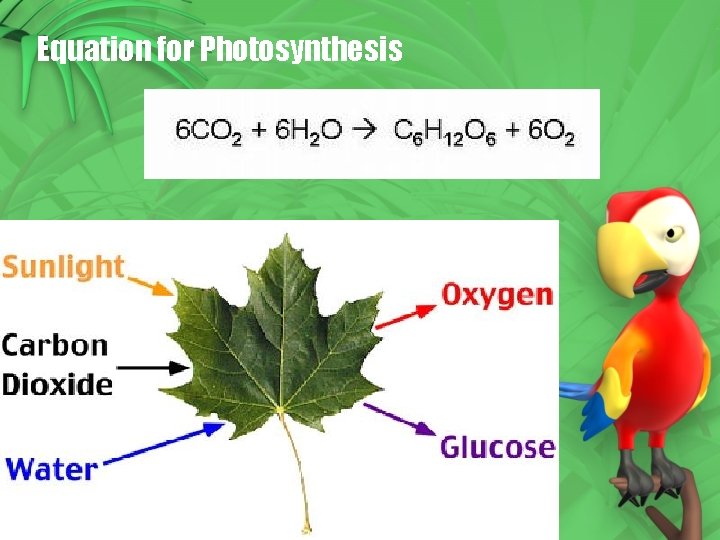
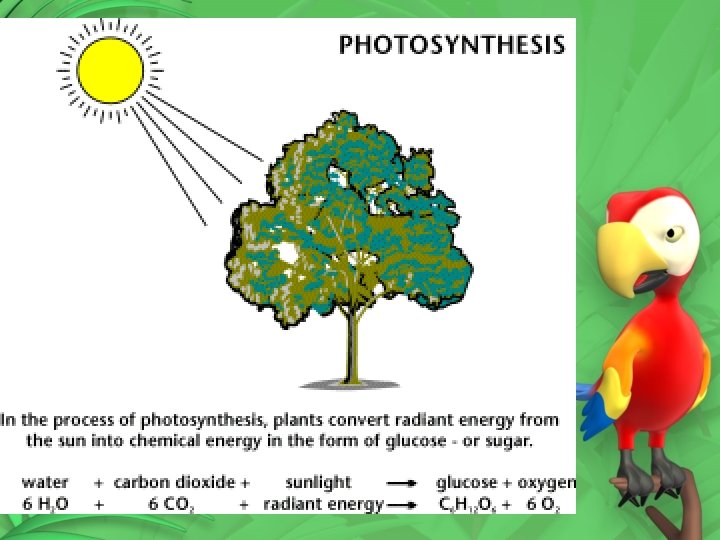
Equation for Photosynthesis



Requirements for Photosynthesis

1. Sunlight • Autotrophs – can use sunlight to make food – Ex. Plants obtain energy • Heterotrophs – obtain energy by eating other organisms – Ex. Animals • All organisms on earth depend on the sun for energy

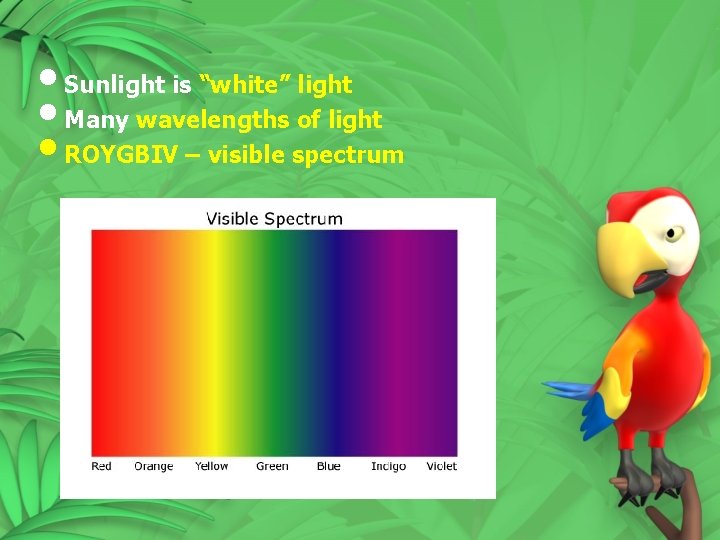
• Sunlight is “white” light • Many wavelengths of light • ROYGBIV – visible spectrum

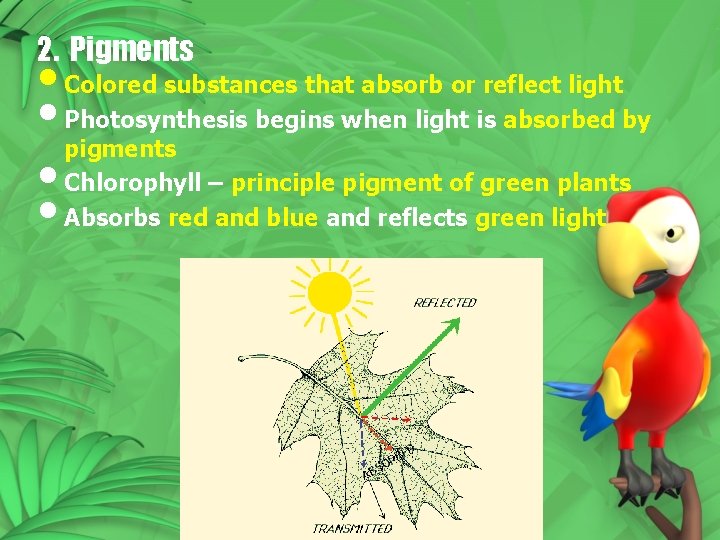
2. Pigments • Colored substances that absorb or reflect light • Photosynthesis begins when light is absorbed by pigments • Chlorophyll – principle pigment of green plants • Absorbs red and blue and reflects green light

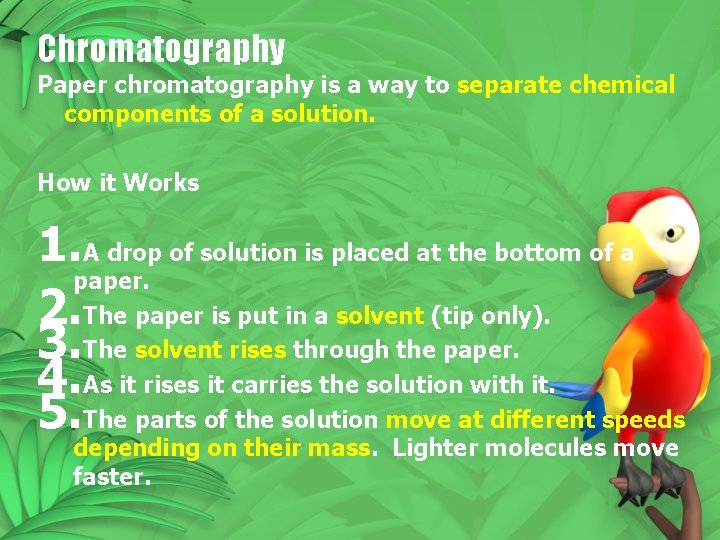
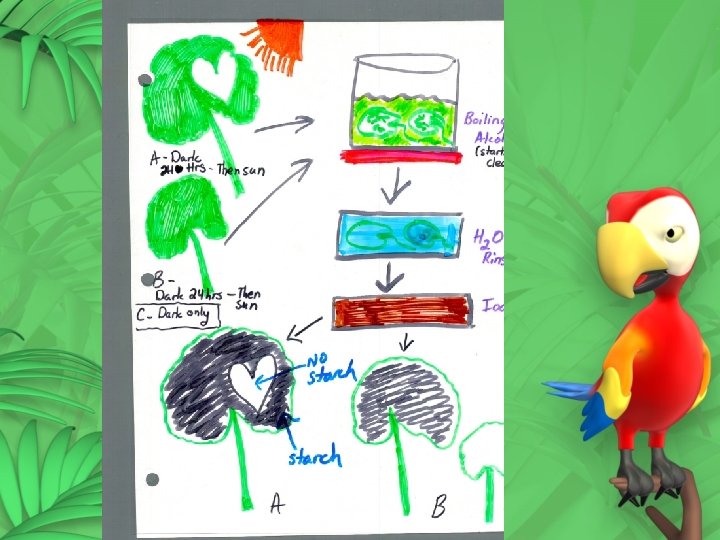
Chromatography Paper chromatography is a way to separate chemical components of a solution. How it Works 1. A drop of solution is placed at the bottom of a paper. 2. The paper is put in a solvent (tip only). 3. The solvent rises through the paper. 4. As it rises it carries the solution with it. 5. The parts of the solution move at different speeds depending on their mass. Lighter molecules move faster.

3. Energy Storing Compounds • Like solar cells • Electrons are raised to higher energy levels – then trapped in bonds • Two ways that energy from the sun is trapped in chemical bonds

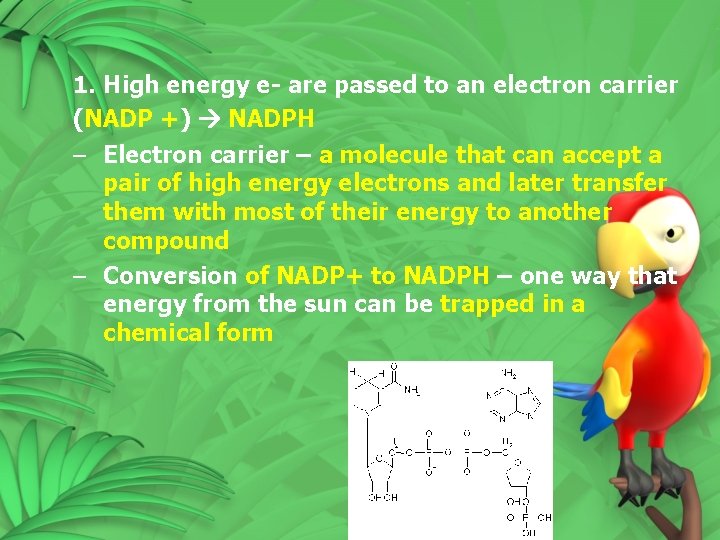
1. High energy e- are passed to an electron carrier (NADP +) NADPH – Electron carrier – a molecule that can accept a pair of high energy electrons and later transfer them with most of their energy to another compound – Conversion of NADP+ to NADPH – one way that energy from the sun can be trapped in a chemical form

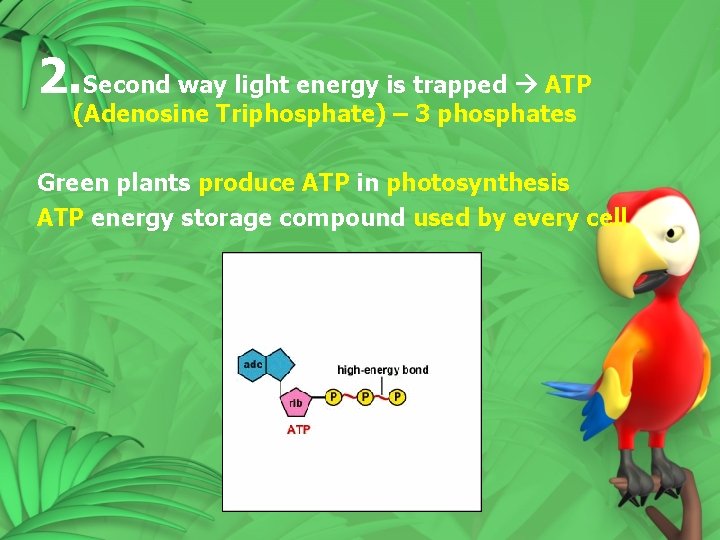
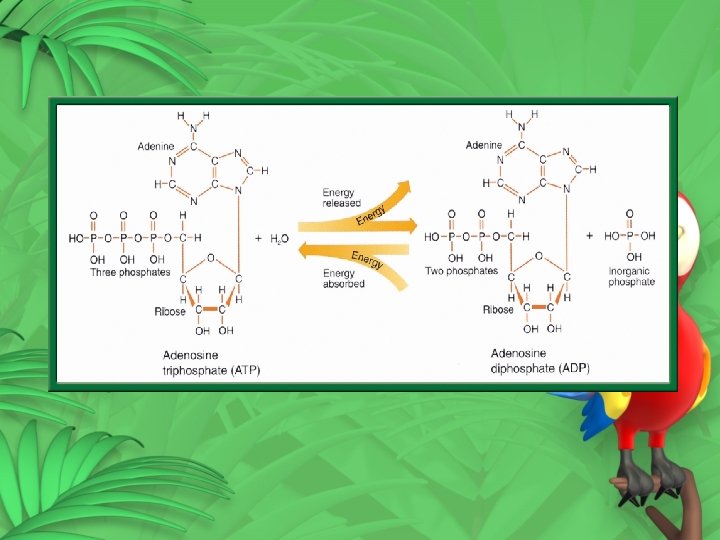
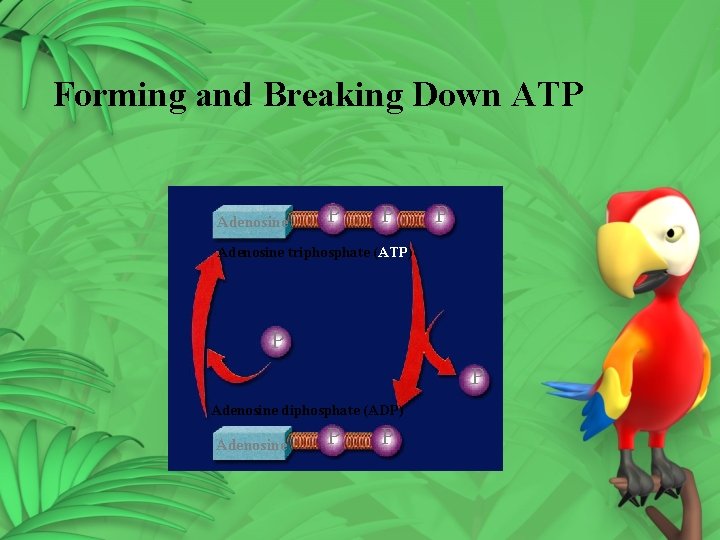
2. Second way light energy is trapped ATP (Adenosine Triphosphate) – 3 phosphates Green plants produce ATP in photosynthesis ATP energy storage compound used by every cell

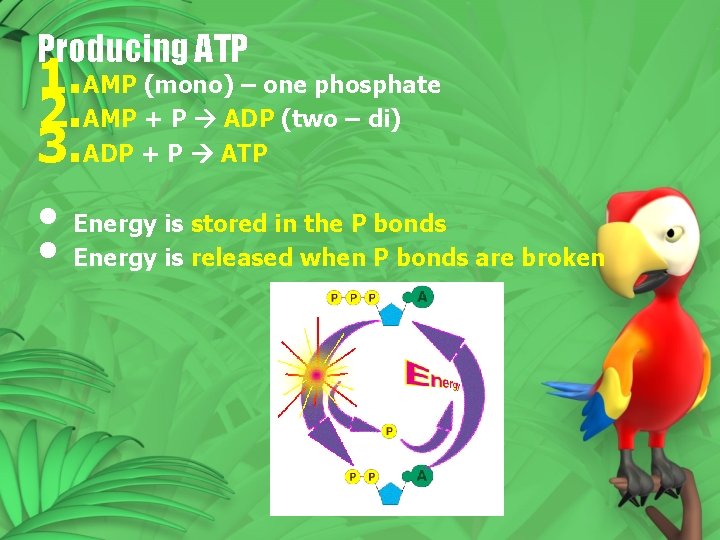
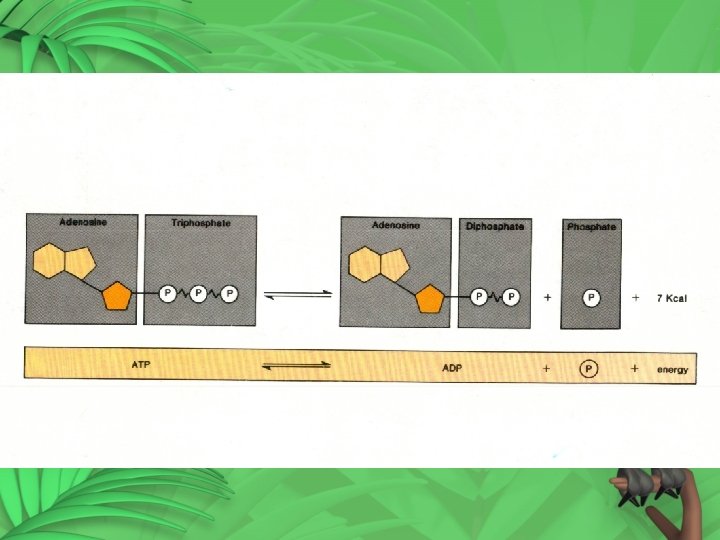
Producing ATP 1. AMP (mono) – one phosphate 2. AMP + P ADP (two – di) 3. ADP + P ATP • Energy is stored in the P bonds • Energy is released when P bonds are broken




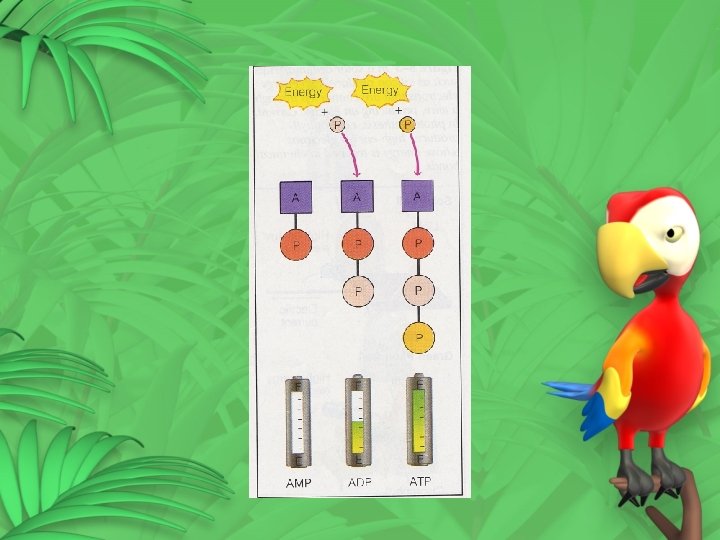
Forming and Breaking Down ATP Adenosine P P P Adenosine triphosphate (ATP) P P Adenosine diphosphate (ADP) Adenosine P P

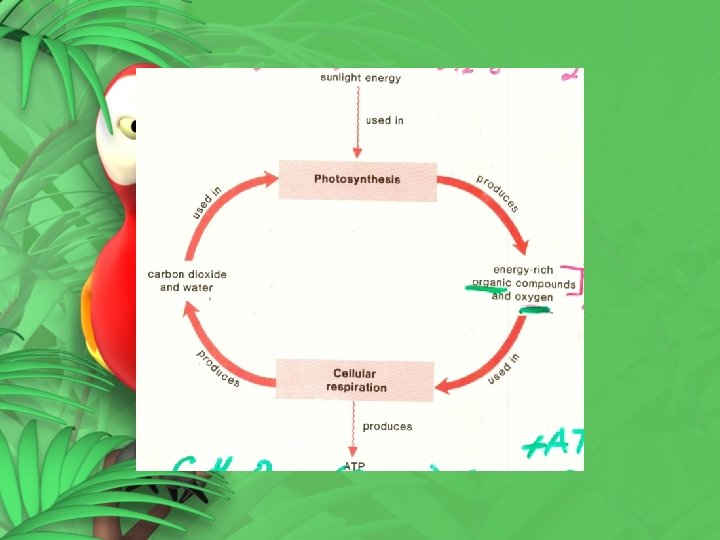
6 -2 Photosynthesis: The Light and Dark Reactions • Light Reaction – energy from sunlight captured to make energy storing compounds • ATP and NADPH • Short term energy storage

• Dark Reaction – energy from ATP and NADPH to make glucose (100 x the energy) • Long term energy storage

The Light Reactions

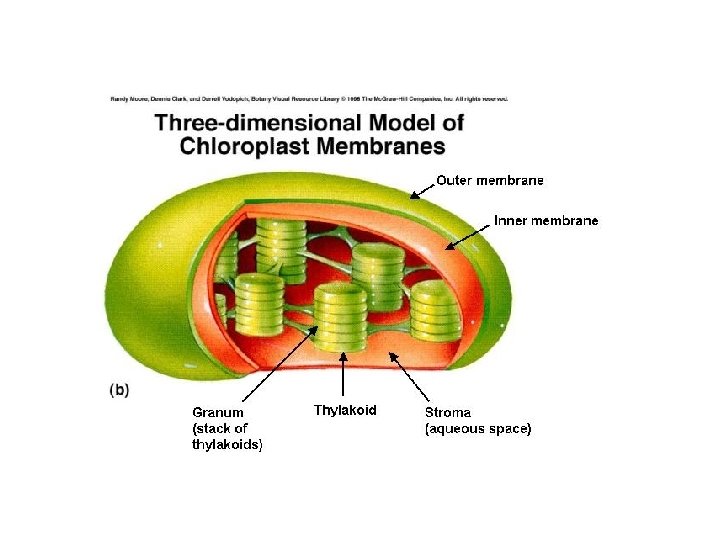
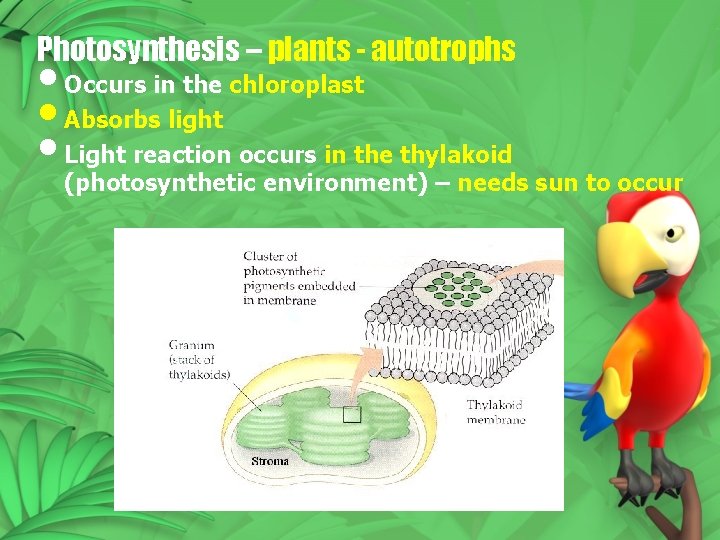
Chloroplast Parts of a chloroplast Stroma – “cytoplasm” Grana – Stack pf pancakes (Thylakoid) Thylakoid – pancakes Thylakoid = photosynthetic membrane


4 Parts of the Light Reaction 1. 2. 3. 4. Light absorption Electron transport Oxygen production ATP formation

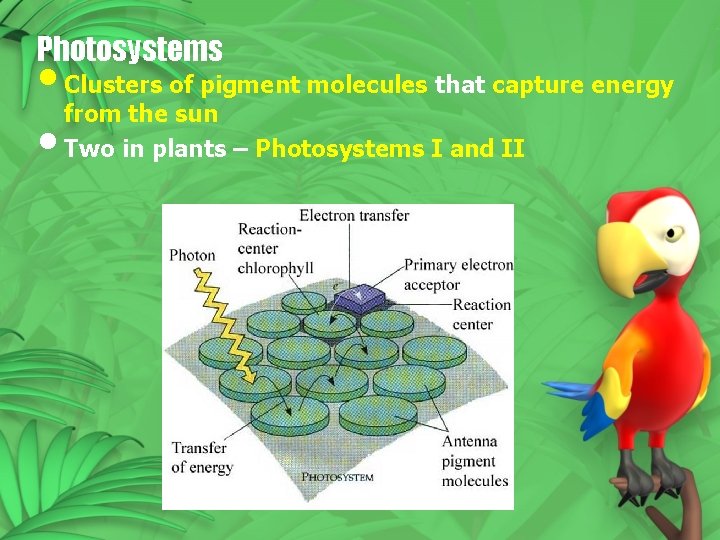
Photosystems • Clusters of pigment molecules that capture energy from the sun • Two in plants – Photosystems I and II

Photosynthesis – plants - autotrophs • Occurs in the chloroplast • Absorbs light • Light reaction occurs in the thylakoid (photosynthetic environment) – needs sun to occur

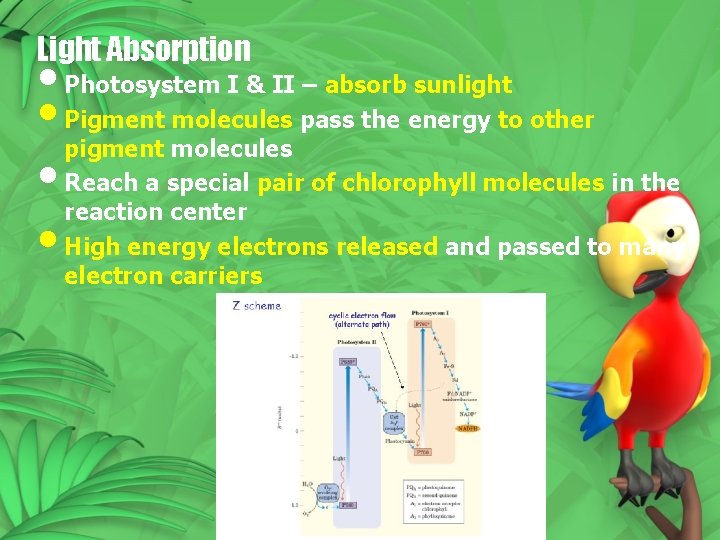
Light Absorption • Photosystem I & II – absorb sunlight • Pigment molecules pass the energy to other pigment molecules • Reach a special pair of chlorophyll molecules in the reaction center • High energy electrons released and passed to many electron carriers

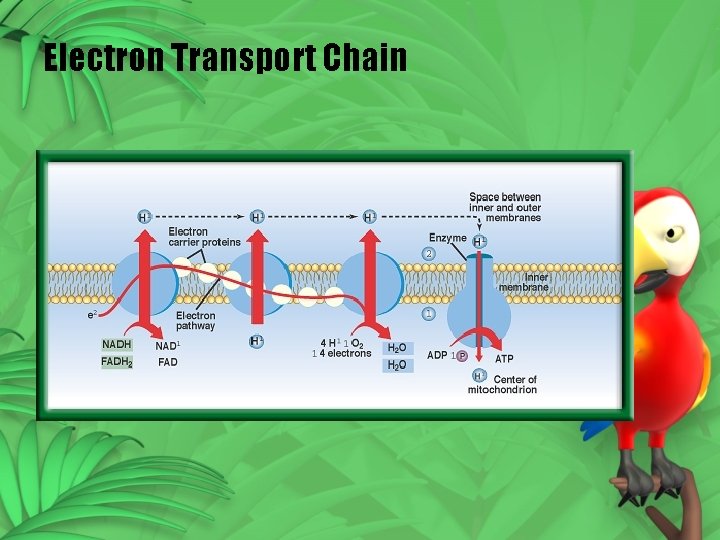
Electron Transport • Electron transport – electron transport chain • e- passed from one carrier to another (bucket brigade) • Passed to electron carrier NADP+ • NADPH

Electron Transport Chain

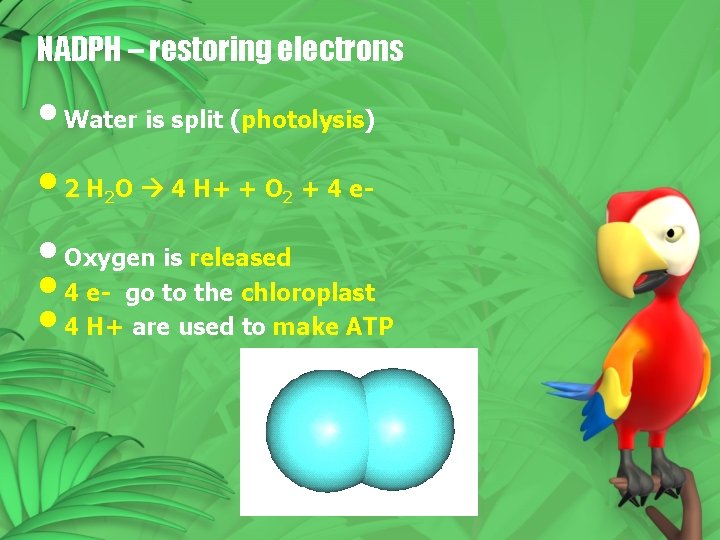
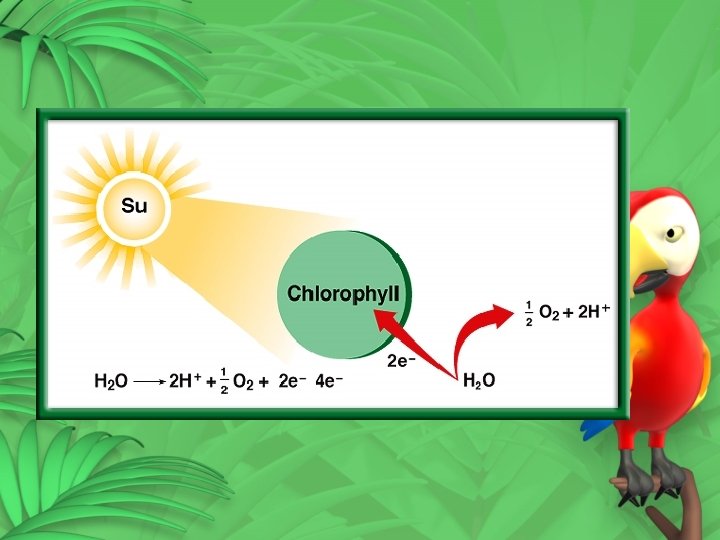
NADPH – restoring electrons • Water is split (photolysis) • 2 H O 4 H+ + O + 4 e • Oxygen is released • 4 e- go to the chloroplast • 4 H+ are used to make ATP 2 2


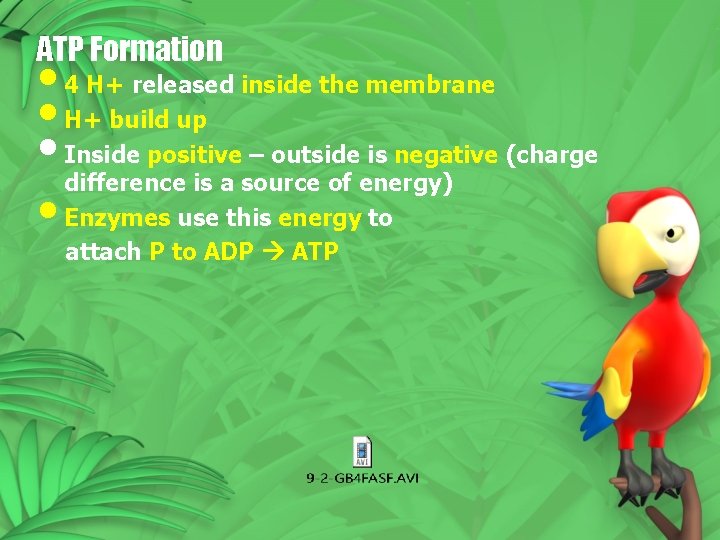
ATP Formation • 4 H+ released inside the membrane • H+ build up • Inside positive – outside is negative (charge difference is a source of energy) • Enzymes use this energy to attach P to ADP ATP


The Dark Reaction or Calvin Cycle

The Dark Reaction or Calvin Cycle • Does not need sunlight to happen • Often happens with sunlight • Uses products of the light reaction (ATP + NADPH) • This series of reactions is particularly critical to living things • Carbon dioxide is used to build complex organic molecules glucose

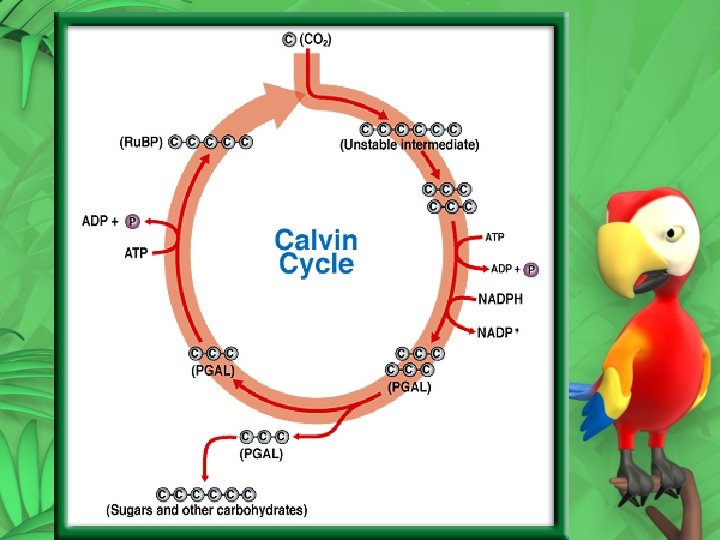
Dark Reaction or Calvin Cycle Occurs in the stroma 5 C sugar (Ru. BP) + CO 2 This reaction is slow and is catalyzed by rubisco Next two 3 C sugars are produced (PGA) ATP and NADPH from the light reaction are used to convert PGA eventually into PGAL (3 C) – products P + ADP and NADP+ PGAL can use some ATP and become Ru. BP (5 C) After several turns of the cycle 2 PGAL can leave and form glucose


6 -3 Glycolysis and Respiration

• Enables organisms to release energy in glucose • Breaks down food molecules • C H O + 6 O 6 CO + 6 H O + energy (ATP) 6 12 6 2 2 2

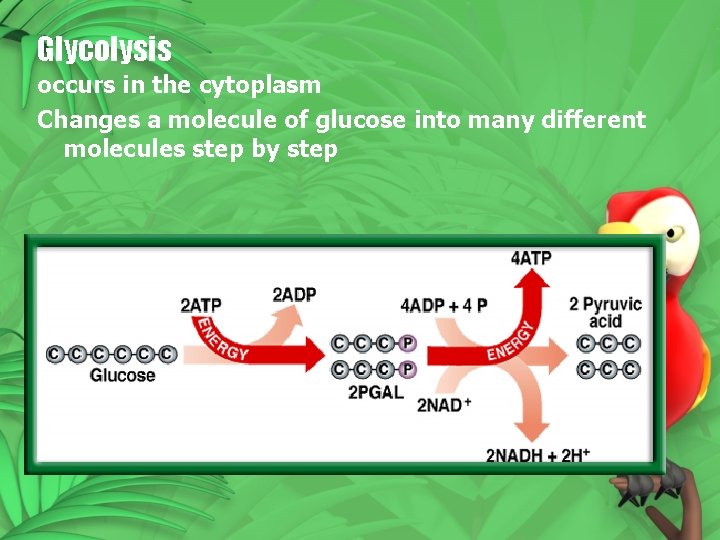
Glycolysis occurs in the cytoplasm Changes a molecule of glucose into many different molecules step by step

• Glucose (6 C) • 2 ATP are used to make 2 -3 -C PGAL • PGAL is converted into pyruvic acid and 4 ATP and 2 NADH are produced • Pyruvic acid can enter aerobic or anaerobic respiration based on whethere is oxygen available or not

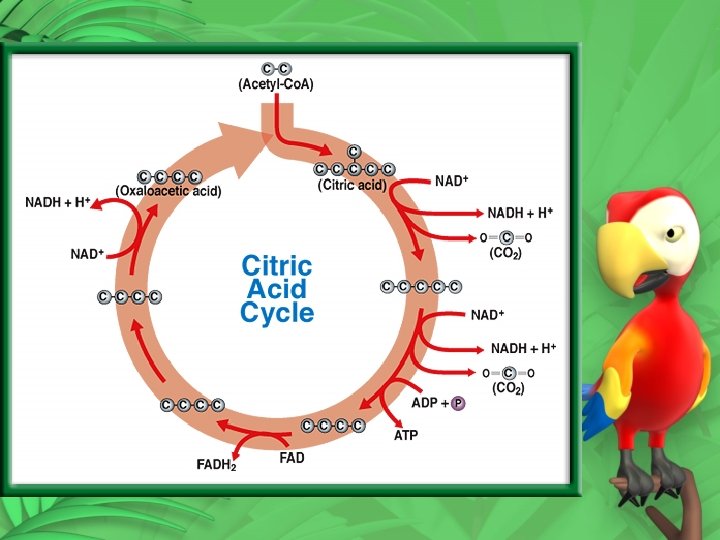
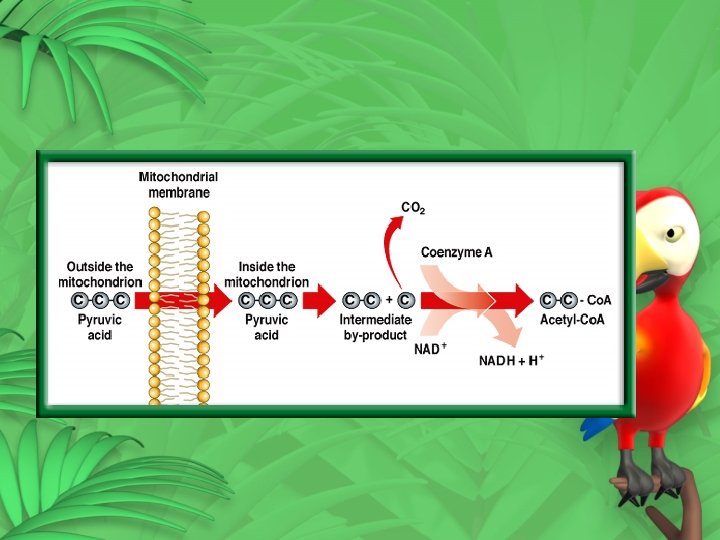
Presence of Oxygen – Cellular Respiration • Aerobic oxygen needed • Takes place in the mitochondria • Krebs cycle (Citric Acid Cycle) • Starts with Pyruvic acid • Carbon dioxide is removed • Acetyl Co. A is produced

• Citric acid is then produced • 9 reactions • 9 intermediates • citric acid is produced and the cycle begins again • Carbon dioxide is released • Make FADH 2 and NADH

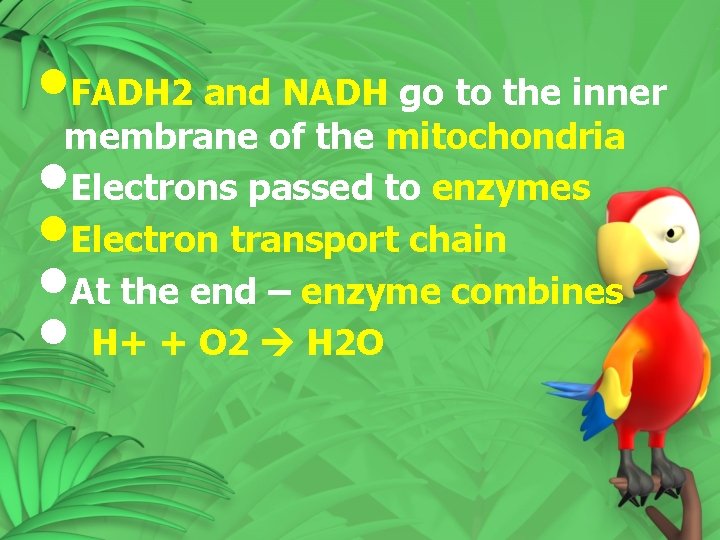
• FADH 2 and NADH go to the inner membrane of the mitochondria • Electrons passed to enzymes • Electron transport chain • At the end – enzyme combines • H+ + O 2 H 2 O

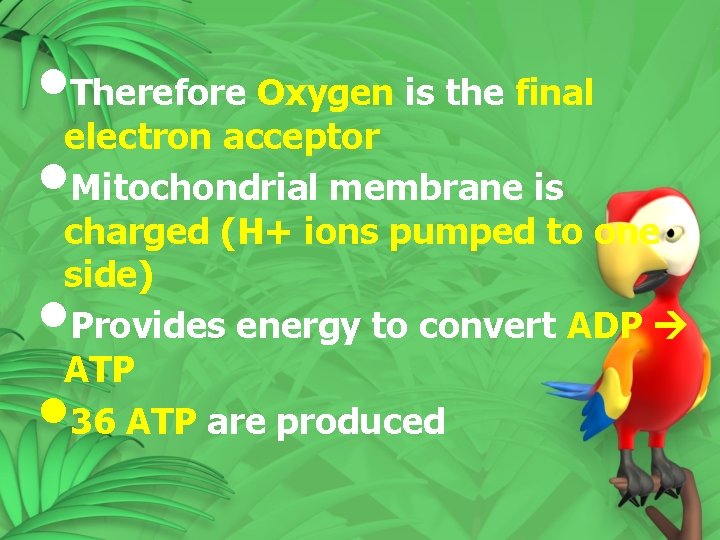
• Therefore Oxygen is the final electron acceptor • Mitochondrial membrane is charged (H+ ions pumped to one side) Provides energy to convert ADP ATP 36 ATP are produced • •





6 -4 Alcoholic Fermentation

• Glycolysis – net 2 ATP NAD+ NADH • If you remove an electron from NADH glycolysis can continue

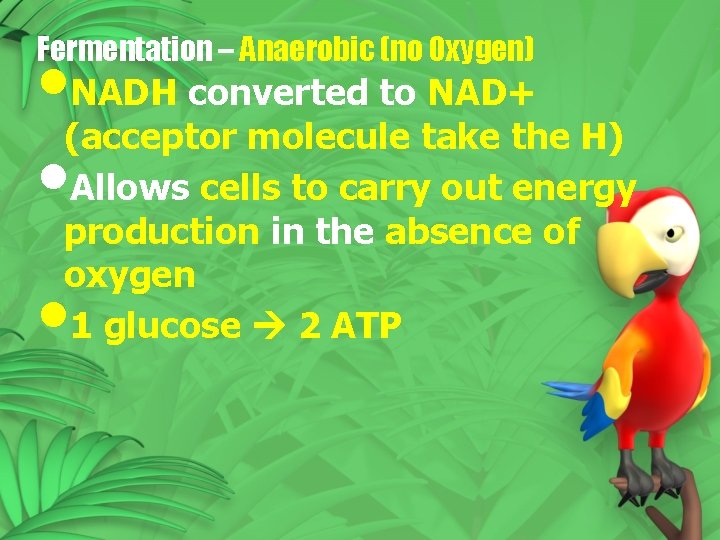
Fermentation – Anaerobic (no Oxygen) • NADH converted to NAD+ (acceptor molecule take the H) • Allows cells to carry out energy production in the absence of oxygen 1 glucose 2 ATP •

• Prokaryotes use many different acceptors • Eukaryotes use two different acceptors 1. Lactic acid fermentation 2. Alcoholic fermentation

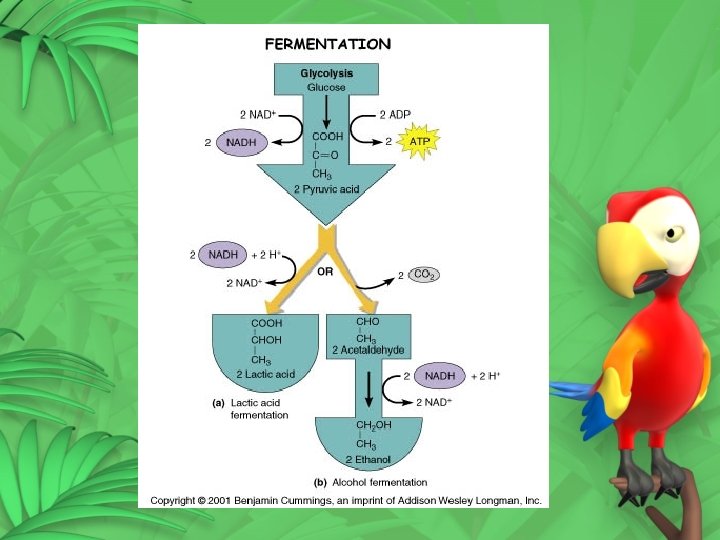
Alcoholic Fermentation Occurs in yeast and a few other organisms Pyruvic acid is broken down to produce 2 -C alcohol and carbon dioxide Pyruvic acid + NADH alcohol + CO 2 + NAD+

Brewers and bakers Carbon dioxide produced causes bread to rise Bubbles in beer Yeast dies at 12% alcohol content

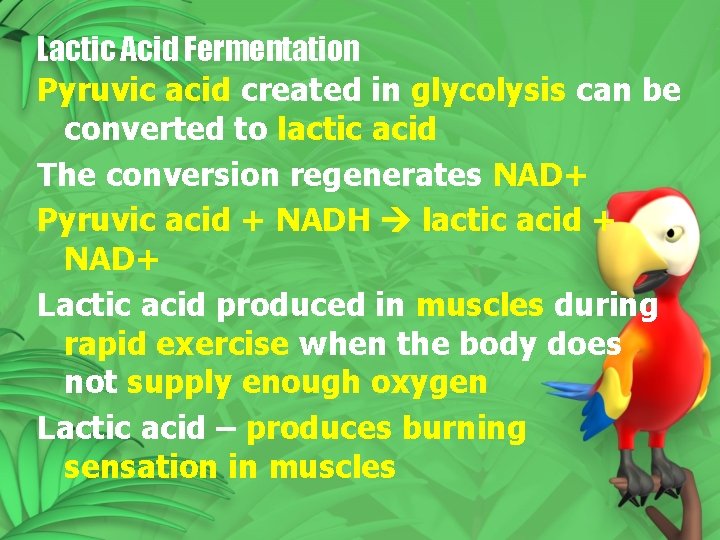
Lactic Acid Fermentation Pyruvic acid created in glycolysis can be converted to lactic acid The conversion regenerates NAD+ Pyruvic acid + NADH lactic acid + NAD+ Lactic acid produced in muscles during rapid exercise when the body does not supply enough oxygen Lactic acid – produces burning sensation in muscles


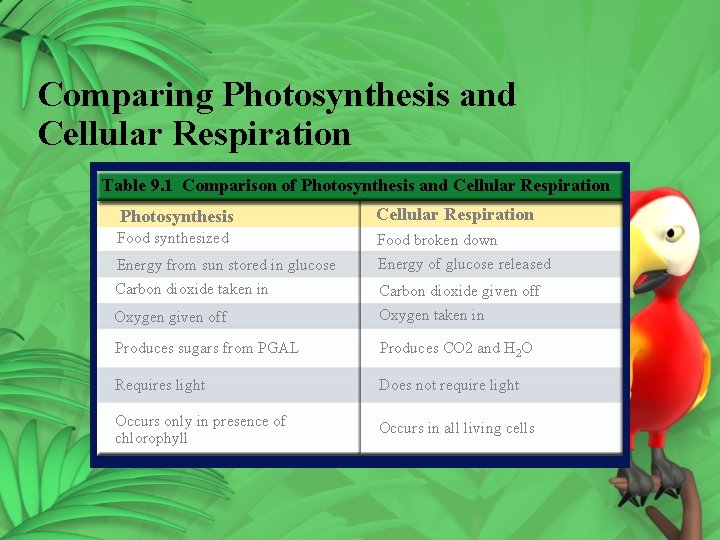
Comparing Photosynthesis and Cellular Respiration Table 9. 1 Comparison of Photosynthesis and Cellular Respiration Photosynthesis Cellular Respiration Food synthesized Food broken down Energy of glucose released Energy from sun stored in glucose Carbon dioxide taken in Oxygen given off Carbon dioxide given off Oxygen taken in Produces sugars from PGAL Produces CO 2 and H 2 O Requires light Does not require light Occurs only in presence of chlorophyll Occurs in all living cells


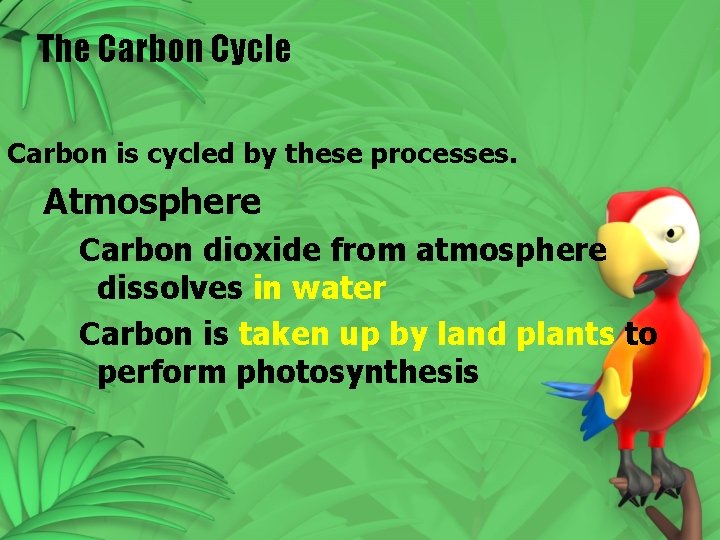
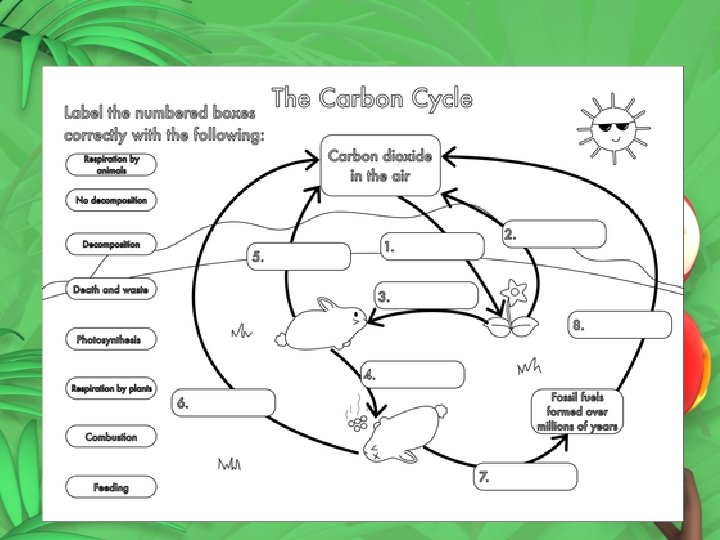
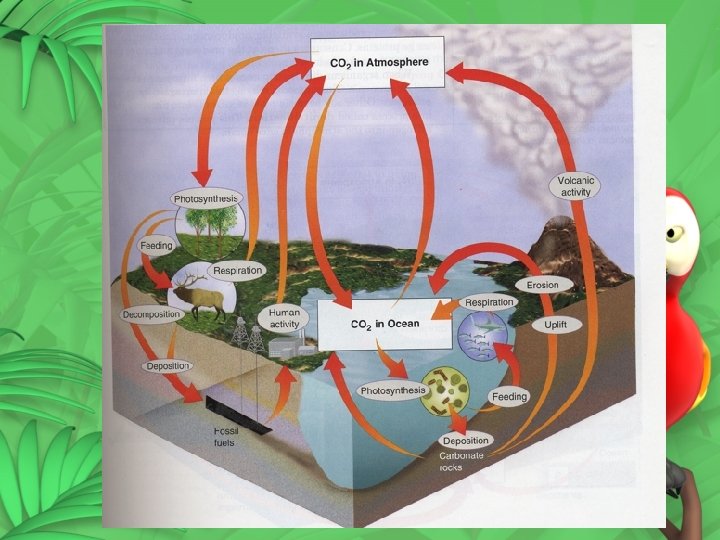
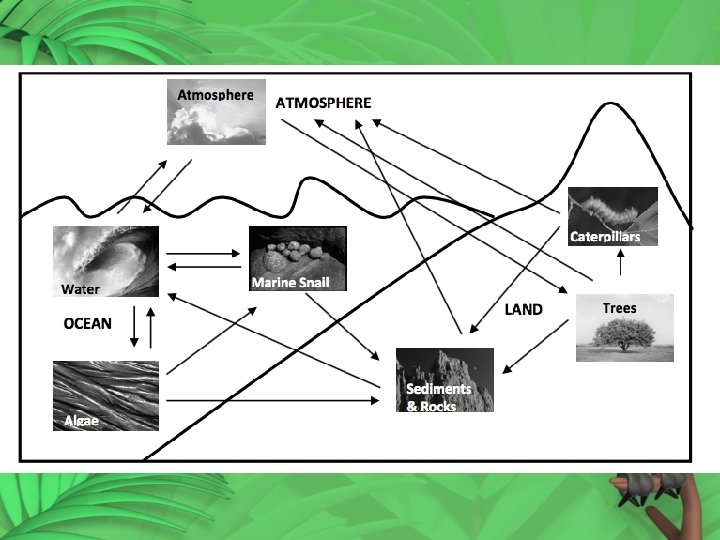
The Carbon Cycle Carbon is cycled by these processes. Atmosphere Carbon dioxide from atmosphere dissolves in water Carbon is taken up by land plants to perform photosynthesis

Water Aquatic plants use carbon to perform photosynthesis Some organisms use carbon from water to build skeletons and shells Carbon dioxide can diffuse from the water back into the atmosphere

Aquatic plants Cellular respiration and decomposition put carbon back into the water Carbon from dead plants can be incorporated into sediments Animals consume aquatic plants and use carbon for energy or store it in their tissues

Aquatic animals Respiration and decomposition put carbon back into the water Carbon from dead animals are incorporated into sediment and can eventually become rocks

Sediments and rocks Weathering and erosion of rocks deposits carbon in rivers and oceans Volcanic eruptions spew carbon containing gasses into the atmosphere

Land plants Cellular respiration and decomposition put carbon back into the atmosphere. Carbon from dead trees can be buried and incorporated into sediments. Plants are consumed by animals that use carbon for energy or store it in their tissues

Land animals Respiration and decomposition of dead animals put carbon back into the atmosphere Carbon from dead animals can be buried and incorporated into sediments.




The End


