Do Now Take out completed homework 1 Take








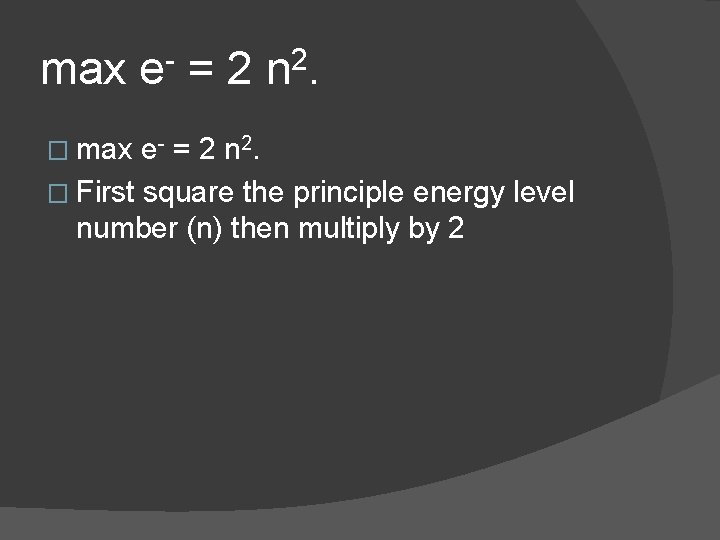




- Slides: 39

Do Now: � Take out completed homework #1 � Take out Atoms Book

Review before the quiz _____1) Bohr’s model of the atom says that electrons a) are mixed in evenly with positive charge b) are found orbiting a positively-charged nucleus c) are found orbiting a positively-charged nucleus in energy levels (shells) d) are found in regions of probability around the nucleus called orbitals

Review Before the Quiz _____5) Dalton’s model of the atom states that atoms e) have positive particles called protons and negative particles called electrons f) have a positively-charged nucleus g) have a positively-charged nucleus with electrons in energy levels (shells) h) are hard solid indivisible spheres

Review Before the Quiz � One model of the atom states that atoms are tiny particles composed of a uniform mixture of positive and negative charges. Scientists conducted an experiment where alpha particles were aimed at a thin layer of gold atoms. Most of the alpha particles passed directly through the gold atoms. A few alpha particles were deflected from their straight-line paths. (This question was taken directly from a Regents Exam).

Review before the quiz � Most of the alpha particles passed directly through the gold atoms undisturbed. What does this evidence suggest about the structure of gold atoms? � Atoms are mostly empty space

Review before the quiz �A few of the alpha particles were deflected. What does this evidence suggest about the structure of the gold atoms? � The nucleus must be positive since it deflects positively charged alpha particles

Quiz-E-Poo time � 12. 5 minutes � Can use your notes/atom book. Turn ALL OF IT IN when you are done � Atom Book � Homework � Quiz

UNIT 4: ELECTRON CONFIGURATION, PERIODIC TRENDS AND HISTORY OF THE ATOM

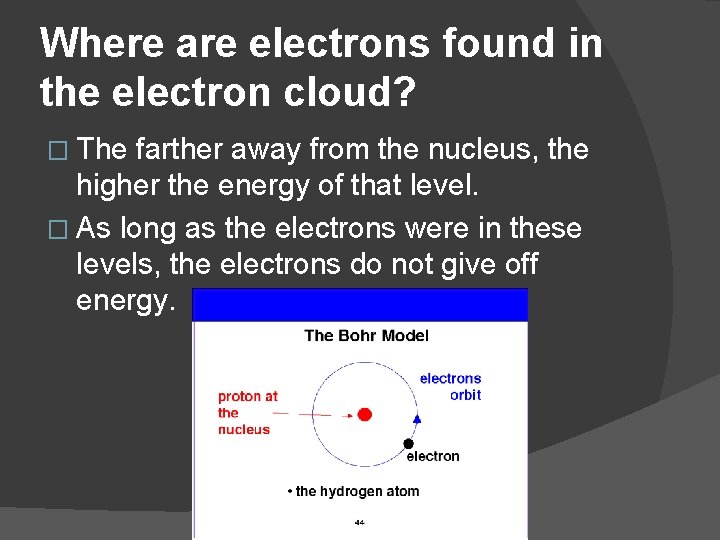
Where are electrons found in the electron cloud? � Bohr was the first to propose that the electrons were located in energy levels � A lower case “n” is used to denote these principle energy levels (also called principle quantum numbers). � The level closest to the nucleus is labeled n = 1. The next level is labeled n = 2 and so forth.

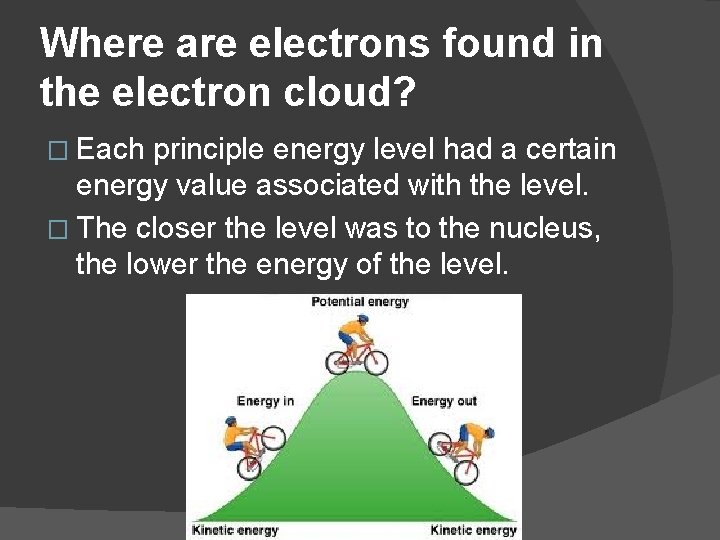
Where are electrons found in the electron cloud? � Each principle energy level had a certain energy value associated with the level. � The closer the level was to the nucleus, the lower the energy of the level.

Where are electrons found in the electron cloud? � The farther away from the nucleus, the higher the energy of that level. � As long as the electrons were in these levels, the electrons do not give off energy.

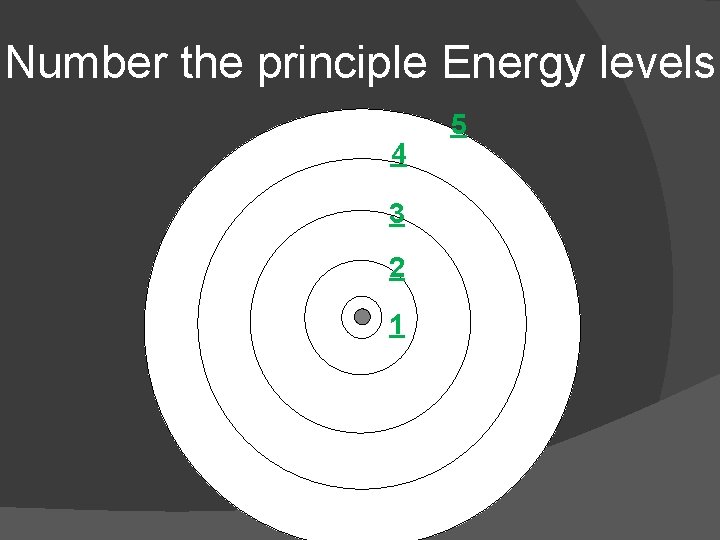
Number the principle Energy levels 4 3 2 1 5

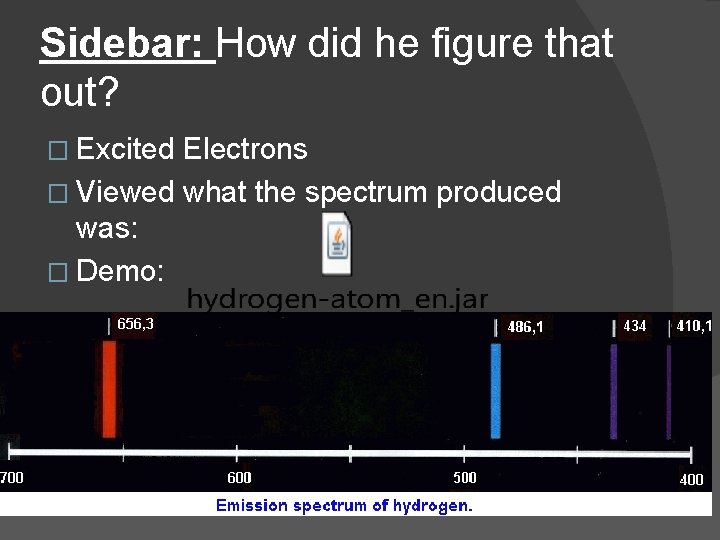
Sidebar: How did he figure that out? � Excited Electrons � Viewed what the spectrum produced was: � Demo:

2. Electron Configuration and the Periodic Table � Each principle energy level can only hold so many electrons before the level is full. � A quick and easy way to determine the maximum number of electrons (max e-) that a principle energy level can hold is given by the following:
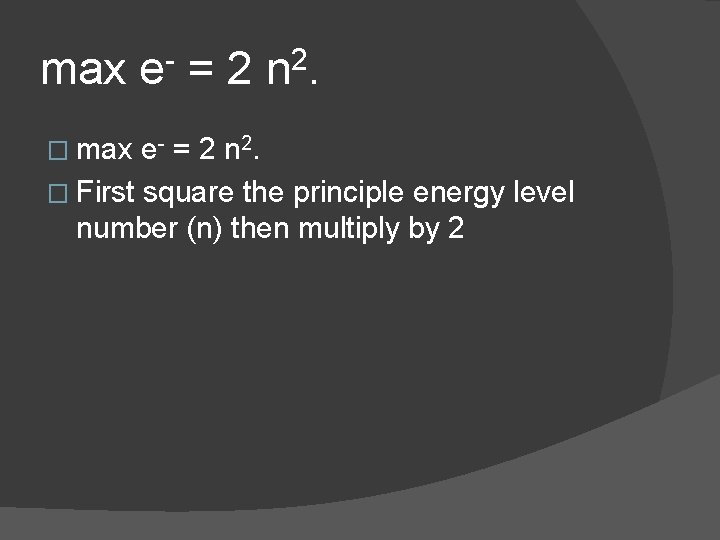
max e- = 2 n 2. � max e - = 2 n 2. � First square the principle energy level number (n) then multiply by 2

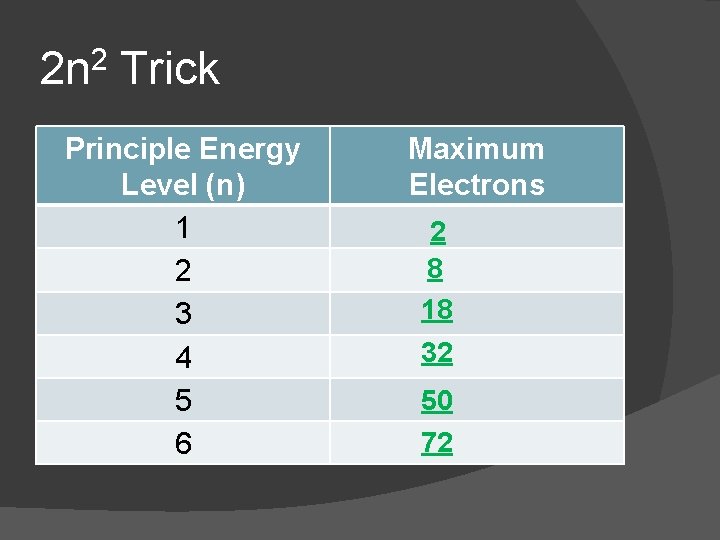
2 n 2 Trick Principle Energy Level (n) 1 2 3 4 5 6 Maximum Electrons 2 8 18 32 50 72

Electron Configuration � Electrons are arranged around the nucleus by filling up the first principle energy level (n=1), then the second energy level, etc. � This is the electron configuration given on your periodic table.

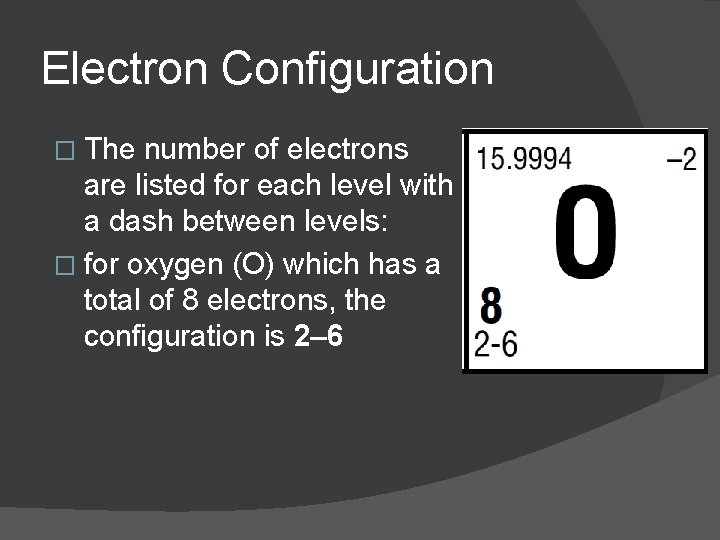
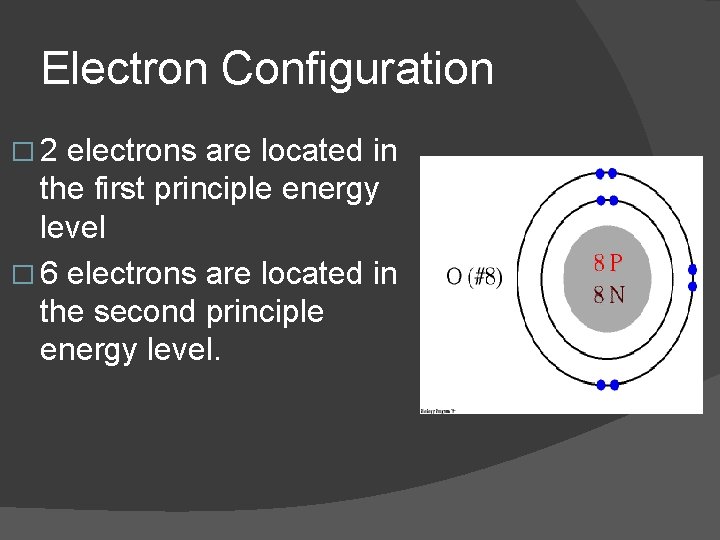
Electron Configuration � The number of electrons are listed for each level with a dash between levels: � for oxygen (O) which has a total of 8 electrons, the configuration is 2– 6

Electron Configuration � 2 electrons are located in the first principle energy level � 6 electrons are located in the second principle energy level.

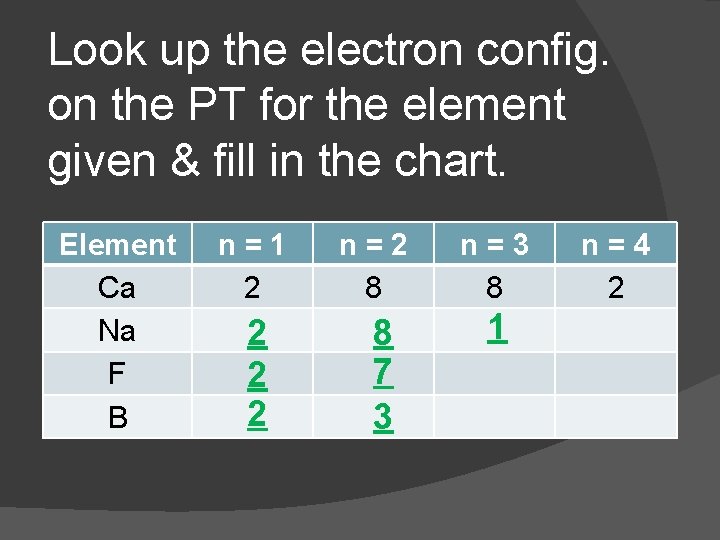
Look up the electron config. on the PT for the element given & fill in the chart. Element Ca Na F B n=1 2 2 n=2 8 8 7 3 n=3 8 1 n=4 2

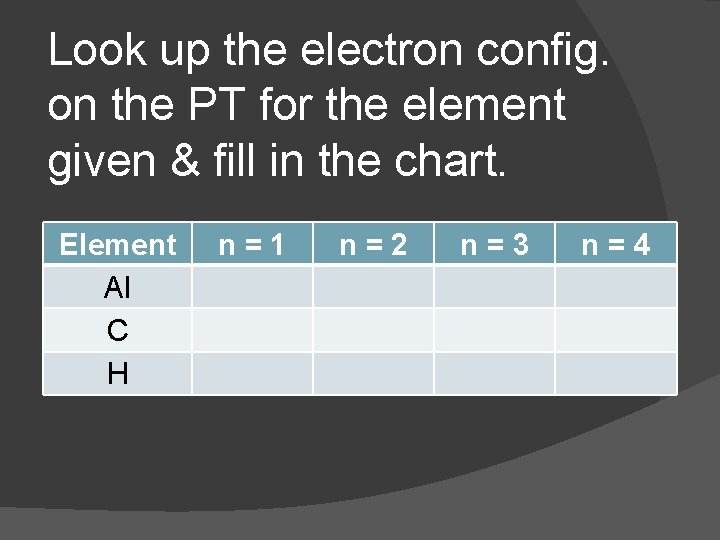
Look up the electron config. on the PT for the element given & fill in the chart. Element Al C H n=1 n=2 n=3 n=4

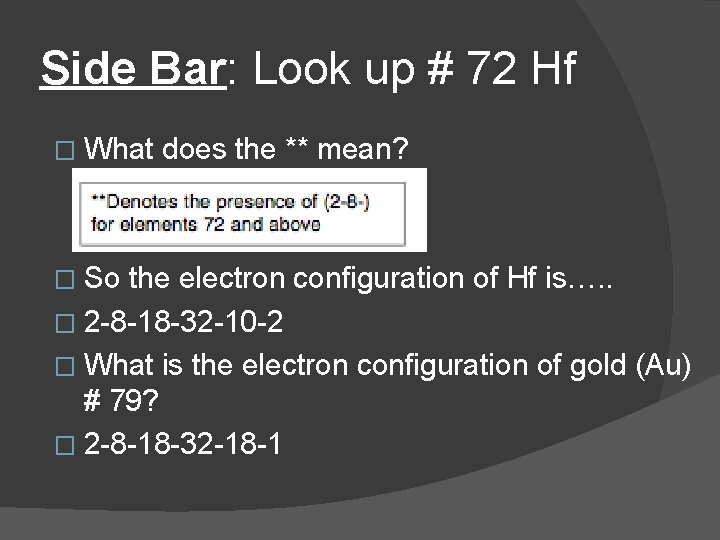
Side Bar: Look up # 72 Hf � What � So does the ** mean? the electron configuration of Hf is…. . � 2 -8 -18 -32 -10 -2 � What is the electron configuration of gold (Au) # 79? � 2 -8 -18 -32 -18 -1

Completely Filled vs. Occupied Principle Energy Levels � Is this room occupied? � Are all the seats completely filled?

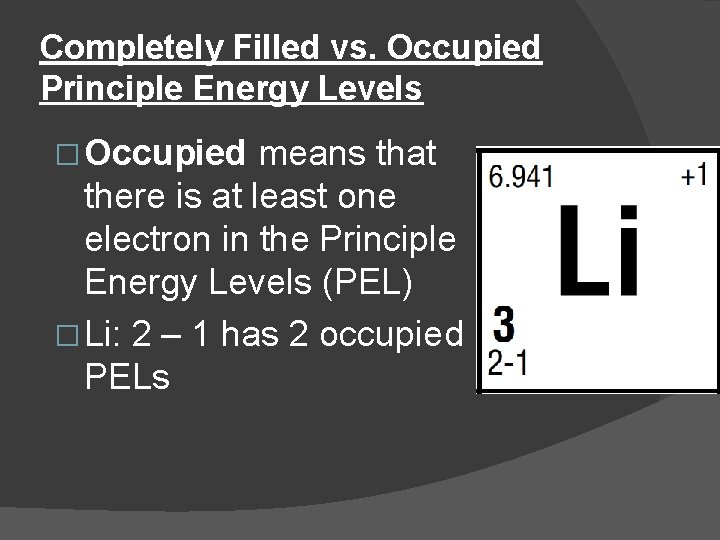
Completely Filled vs. Occupied Principle Energy Levels � Occupied means that there is at least one electron in the Principle Energy Levels (PEL) � Li: 2 – 1 has 2 occupied PELs

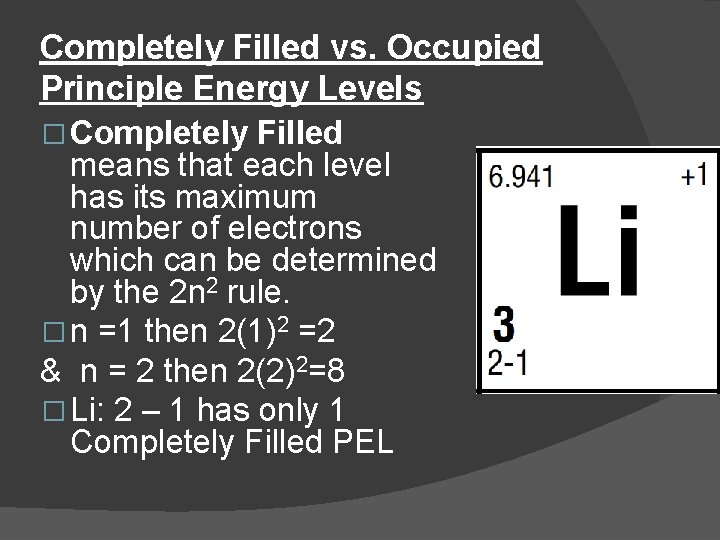
Completely Filled vs. Occupied Principle Energy Levels � Completely Filled means that each level has its maximum number of electrons which can be determined by the 2 n 2 rule. � n =1 then 2(1)2 =2 & n = 2 then 2(2)2=8 � Li: 2 – 1 has only 1 Completely Filled PEL

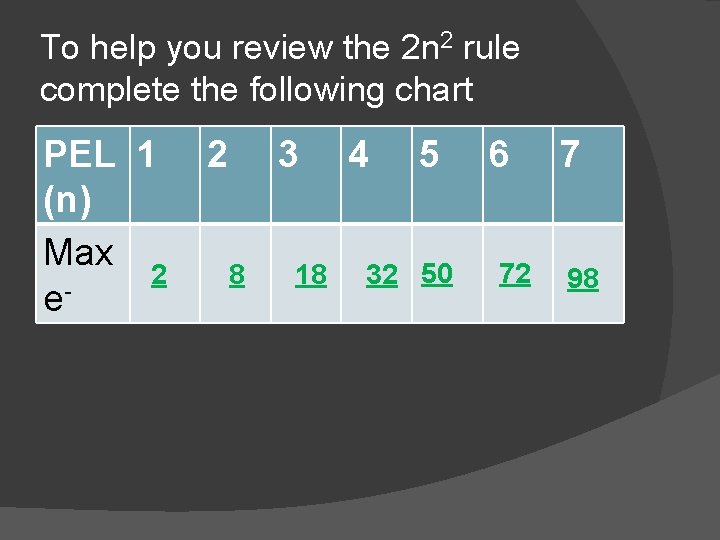
To help you review the 2 n 2 rule complete the following chart PEL 1 (n) Max 2 e- 2 3 8 18 4 5 32 50 6 72 7 98

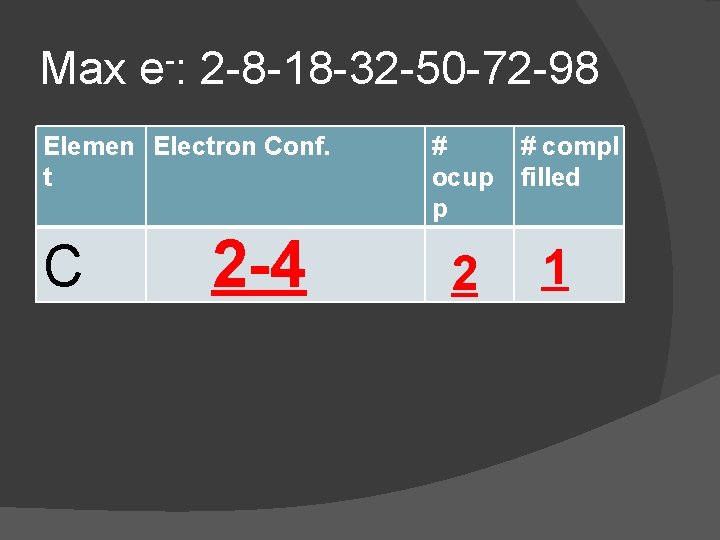
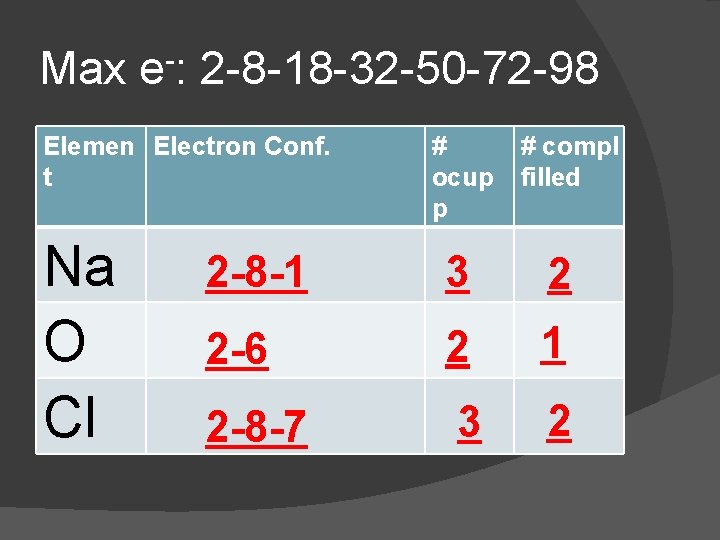
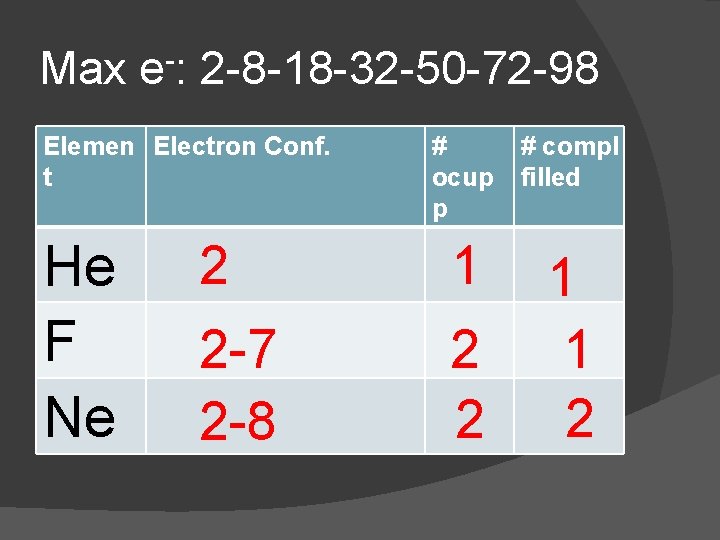
In the chart: � Copy the electron configuration from the Periodic Table � Determine the number of Occupied Principle Energy Levels (PEL) � Determine the number of Completely Filled Principle Energy Levels

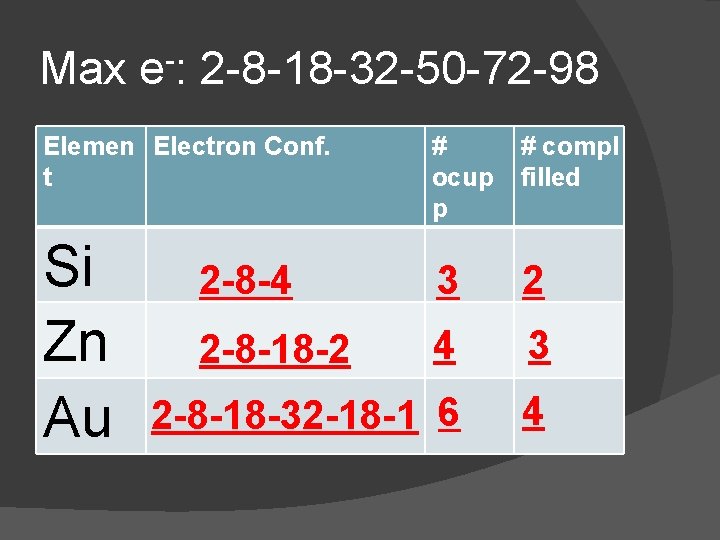
Max e-: 2 -8 -18 -32 -50 -72 -98 Elemen Electron Conf. t C 2 -4 # ocup p 2 # compl filled 1

Max e-: 2 -8 -18 -32 -50 -72 -98 Elemen Electron Conf. t Na O Cl # ocup p # compl filled 2 -8 -1 3 2 2 -6 2 1 2 -8 -7 3 2

Max e-: 2 -8 -18 -32 -50 -72 -98 Elemen Electron Conf. t He F Ne # ocup p # compl filled 2 1 2 -7 2 -8 2 2 1 1 2

Max e-: 2 -8 -18 -32 -50 -72 -98 Elemen Electron Conf. t Si Zn Au # ocup p # compl filled 2 -8 -4 3 2 2 -8 -18 -2 4 3 2 -8 -18 -32 -18 -1 6 4

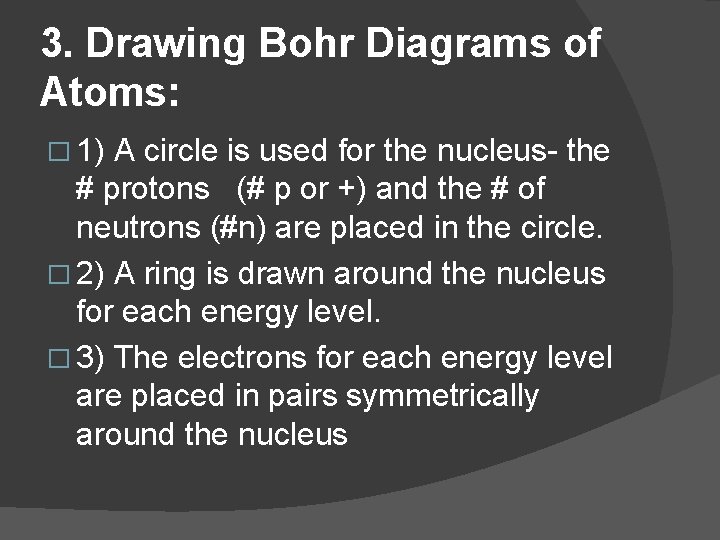
3. Drawing Bohr Diagrams of Atoms: � 1) A circle is used for the nucleus- the # protons (# p or +) and the # of neutrons (#n) are placed in the circle. � 2) A ring is drawn around the nucleus for each energy level. � 3) The electrons for each energy level are placed in pairs symmetrically around the nucleus

For Fluorine (F) atomic # = _____ atomic mass = ______ electron configuration: _________ _ #p= _______ #n =________

For Aluminum (Al) atomic # = _____ atomic mass = ______ electron configuration: _________ #p= _______ #n =________

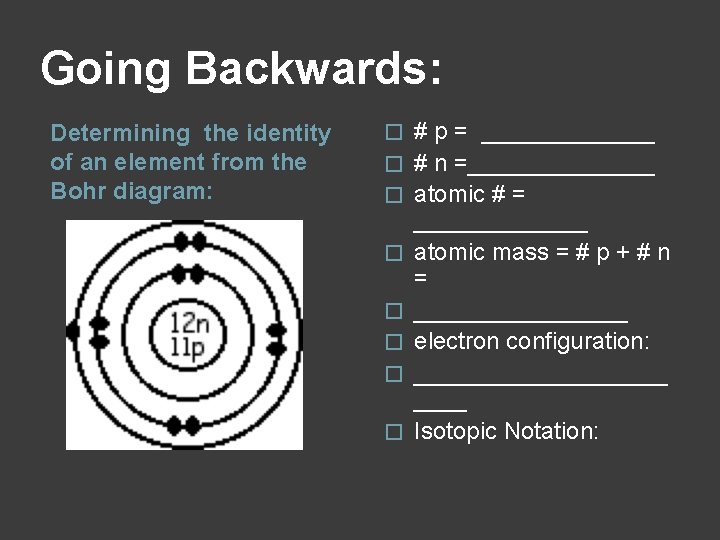
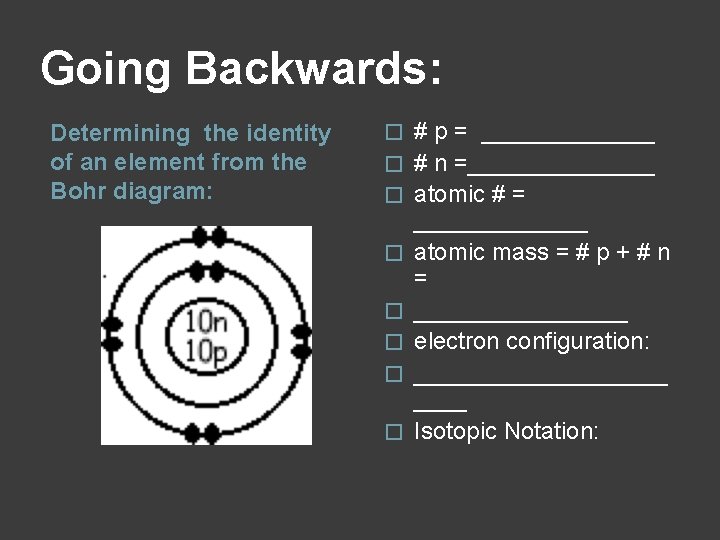
Going Backwards: Determining the identity of an element from the Bohr diagram: � � � � # p = _______ # n =_______ atomic # = _______ atomic mass = # p + # n = ________ electron configuration: __________ Isotopic Notation:

Going Backwards: Determining the identity of an element from the Bohr diagram: � � � � # p = _______ # n =_______ atomic # = _______ atomic mass = # p + # n = ________ electron configuration: __________ Isotopic Notation:

To review: To draw a Bohr Model: � Look up the atomic #, atomic mass & the electron configuration � Determine the # of neutrons (atomic mass-atomic #) � Draw nucleus & write the # p & #n in nucleus � Draw rings around the nucleus equal to the # of PEL in atom (# of numbers in electron configuration) � Place electrons for each level symmetrically in the rings in pairs �

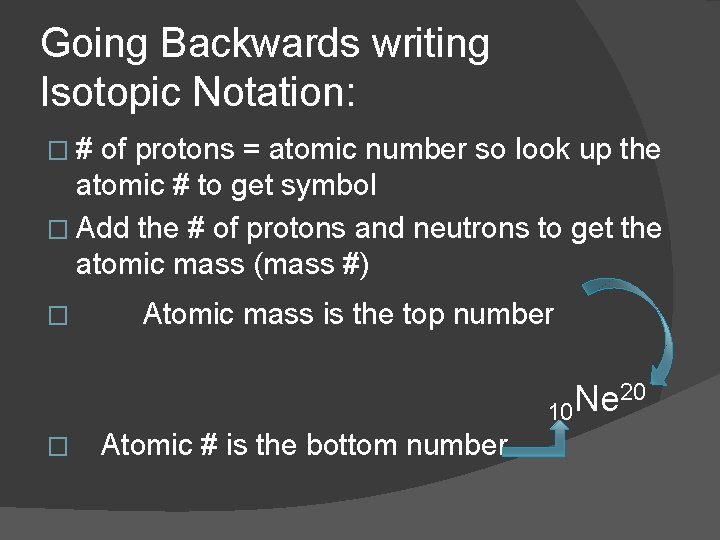
Going Backwards writing Isotopic Notation: �# of protons = atomic number so look up the atomic # to get symbol � Add the # of protons and neutrons to get the atomic mass (mass #) � Atomic mass is the top number 20 Ne 10 � Atomic # is the bottom number

Now it is your turn! Complete the Bohr Model worksheet. � When done, show me and turn in. � If not done, it becomes homework along with homework #2. �