DO NOW Sketch a graph of temperature vs








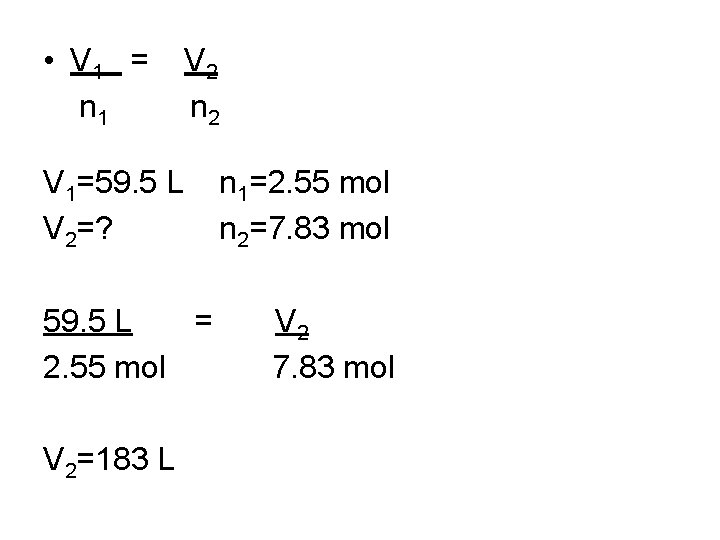

- Slides: 15

DO NOW: Sketch a graph of temperature vs. volume of a gas at constant pressure. Sketch a graph of pressure vs. volume of a gas at constant temperature.

Aim: How are pressure and temperature of a gas related? How are volume and number of moles of a gas related?

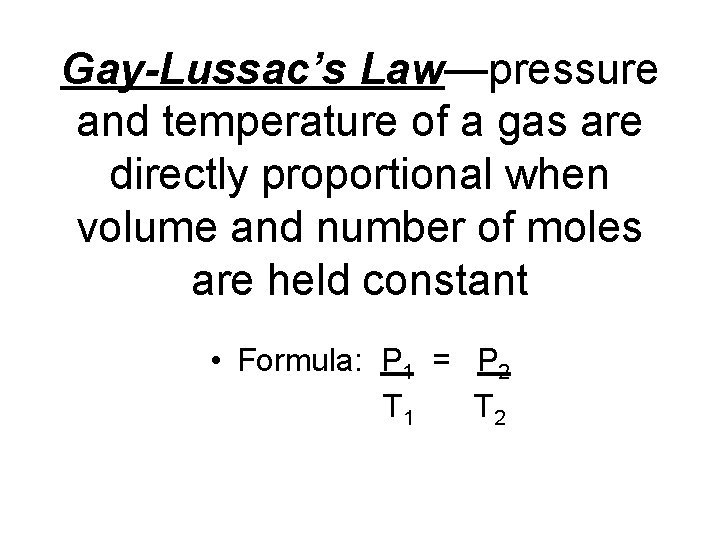
Gay-Lussac’s Law—pressure and temperature of a gas are directly proportional when volume and number of moles are held constant • Formula: P 1 = P 2 T 1 T 2

The pressure exerted by a gas in an immovable piston at 273 K is 99. 7 k. Pa. What will be the pressure of this gas when the temperature is decreased to 200. K?

• P 1 = P 2 T 1 T 2 P 1=99. 7 k. Pa P 2=? T 1=273 K T 2=200. K 99. 7 k. Pa = P 2 273 K 200. K P 2=73. 0 k. Pa

The pressure exerted by a gas is 9. 4 atm. After the temperature is increased to 77 C, the pressure of the gas is 15. 2 atm. What is the initial temperature of this system?

• P 1 = P 2 T 1 T 2 P 1=9. 4 atm T 1=? P 2=15. 2 atm T 2=350. K 9. 4 atm = 15. 2 atm T 1 350. K T 1=220 K

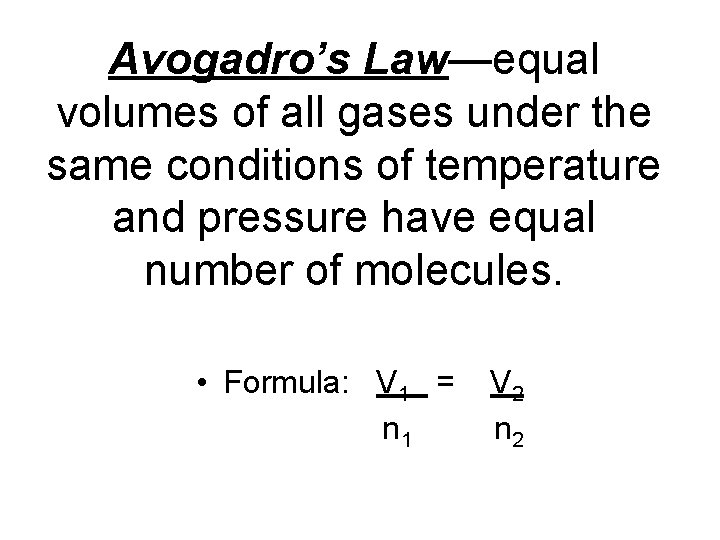
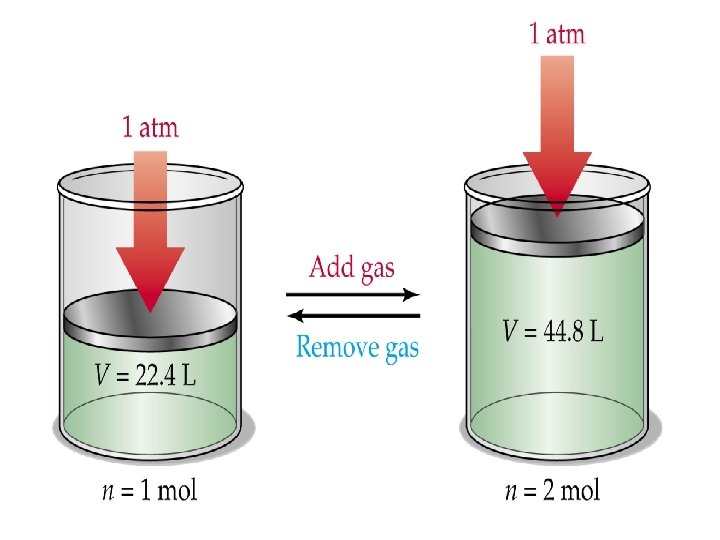
Avogadro’s Law—equal volumes of all gases under the same conditions of temperature and pressure have equal number of molecules. • Formula: V 1 = n 1 V 2 n 2


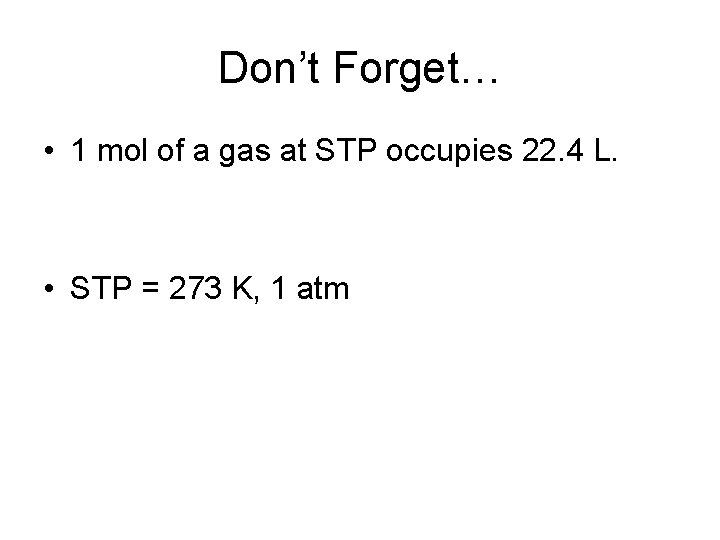
Don’t Forget… • 1 mol of a gas at STP occupies 22. 4 L. • STP = 273 K, 1 atm

A sample of Ar occupies 67. 2 L at STP. How many moles of the gas are present? • V 1 = n 1 V 1=22. 4 L V 2=67. 2 L n 2=3 mol V 2 n 1=1 mol n 2=?

If 2. 55 mol of He gas occupies a volume of 59. 5 L at a particular temperature and pressure, what volume does 7. 83 mol of He occupy under the same conditions?
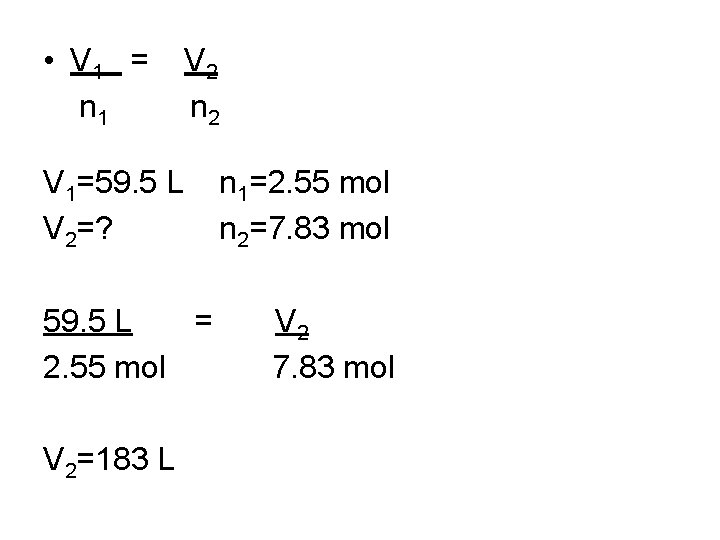
• V 1 = n 1 V 2 n 2 V 1=59. 5 L V 2=? 59. 5 L = 2. 55 mol V 2=183 L n 1=2. 55 mol n 2=7. 83 mol V 2 7. 83 mol

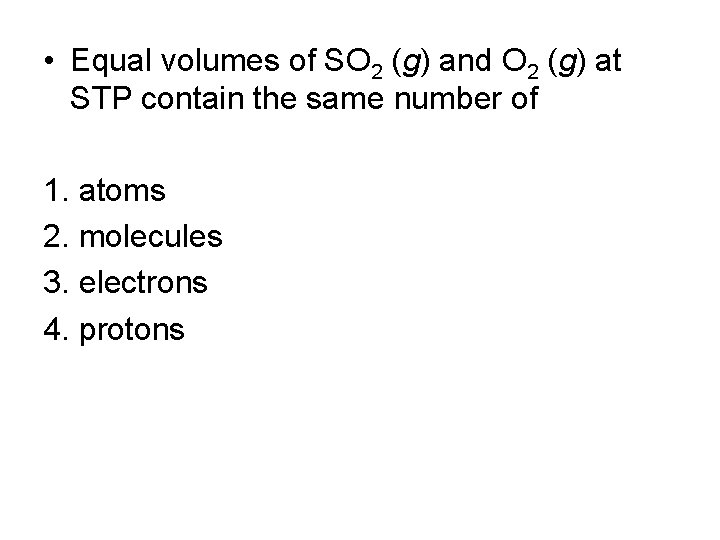
• Equal volumes of SO 2 (g) and O 2 (g) at STP contain the same number of 1. atoms 2. molecules 3. electrons 4. protons

• A sample of oxygen gas is sealed in container X. A sample of hydrogen gas is sealed in container Z. Both samples have the same volume, temperature, and pressure. Which statement is true? 1. Container X contains more gas molecules than container Z. 2. Container X contains fewer gas molecules than container Z. 3. Containers X and Z both contain the same number of gas molecules. 4. Containers X and Z both contain the same mass of gas.