DO NOW Record and Answer these questions 1

























- Slides: 52

DO NOW �Record and Answer these questions 1. What caused Kali’s brain infection? 2. Where did she contract her infection? 3. How many people survive this infection? http: //www. youtube. com/watch? v=D 2 gg. Jh. EKIi c

DO NOW �Gather with your Quicker Picker Upper Group �You have 5 min to refresh and prepare for presentation

Chemistry of Life

Hierarchy of Living Things �Atom Molecule Cell Tissue Organ System Organism

Common Elements �Element- made of only 1 type of atom � 98% of all living things are made of… �C H O N P S

MOLECULES Connections of atoms that have unfilled outer rings

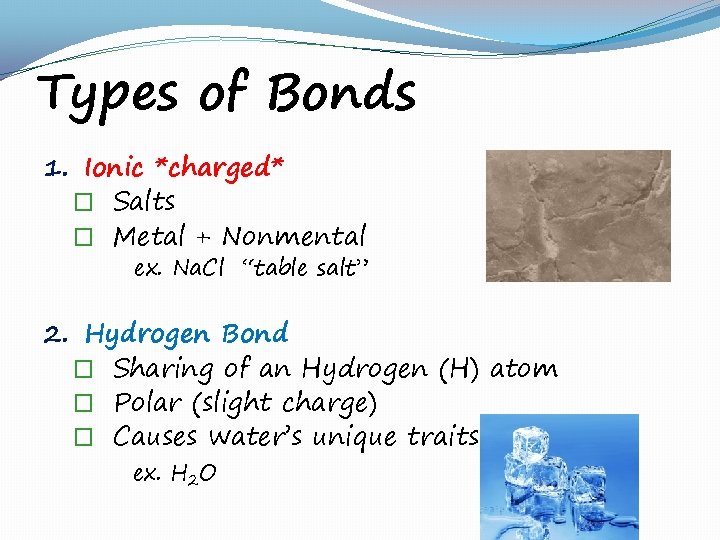
Types of Bonds 1. Ionic *charged* � Salts � Metal + Nonmental ex. Na. Cl “table salt” 2. Hydrogen Bond � Sharing of an Hydrogen (H) atom � Polar (slight charge) � Causes water’s unique traits ex. H 2 O

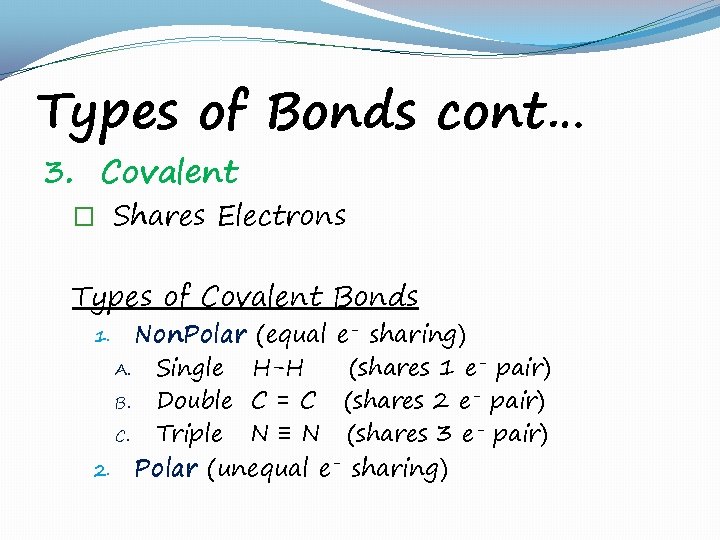
Types of Bonds cont… 3. Covalent � Shares Electrons Types of Covalent Bonds Non. Polar (equal e- sharing) A. Single H-H (shares 1 e- pair) B. Double C = C (shares 2 e- pair) C. Triple N ≡ N (shares 3 e- pair) 2. Polar (unequal e- sharing) 1.

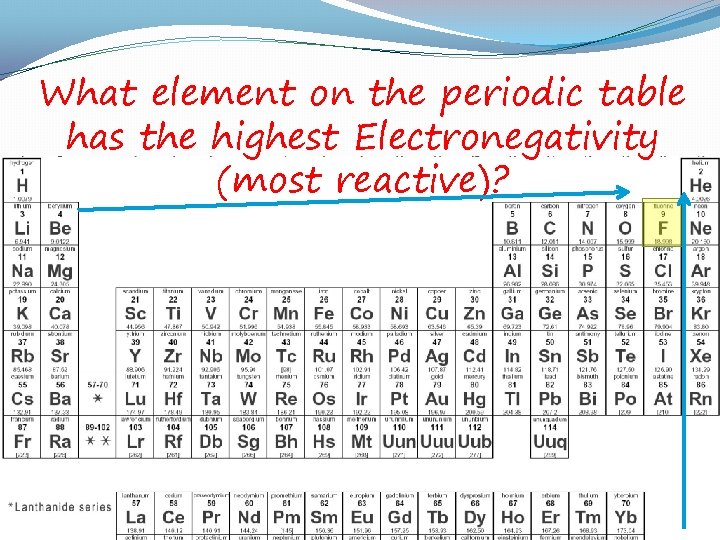
What element on the periodic table has the highest Electronegativity (most reactive)?

Electronegativity �The pull of electrons towards nucleus 1. NONPOLAR- 2 atoms with similar Electroneg. � No charge, shares e- equally 2. POLAR- unequal sharing of atoms � Slightly charged � Ex. H 2 O, O steals more of the ethan the H’s H O H

Water’s Unique Properties

Water’s Unique Traits 1. Cohesion (sticks to itself & others) 2. Universal Solvent 3. Resists Temperature Change 4. Liquid is denser(heavier) than solid http: //www. youtube. com/watch? v=p S 7 Q 4 p. Nm 1 HM

Do Now Without looking at your notes, fill in “CURL”Water’s Unique Properties

Functional Groups

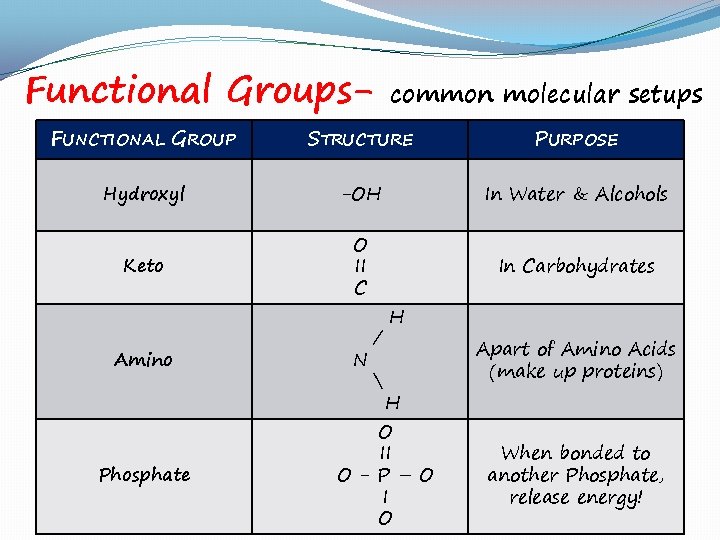
Functional Groups- common molecular setups FUNCTIONAL GROUP STRUCTURE PURPOSE Hydroxyl -OH In Water & Alcohols Keto O II C In Carbohydrates Amino Phosphate N / H Apart of Amino Acids (make up proteins) H O II O- P–O I O When bonded to another Phosphate, release energy!

Macromolecules

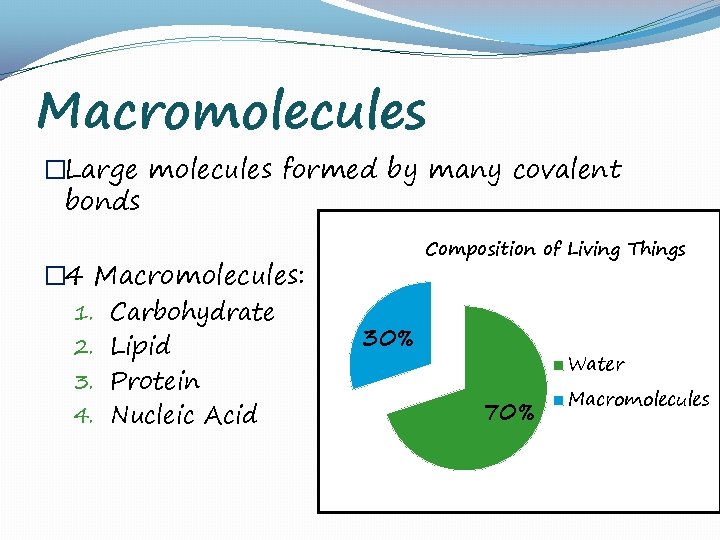
Macromolecules �Large molecules formed by many covalent bonds � 4 Macromolecules: 1. Carbohydrate 2. Lipid 3. Protein 4. Nucleic Acid Composition of Living Things 30% Water 70% Macromolecules

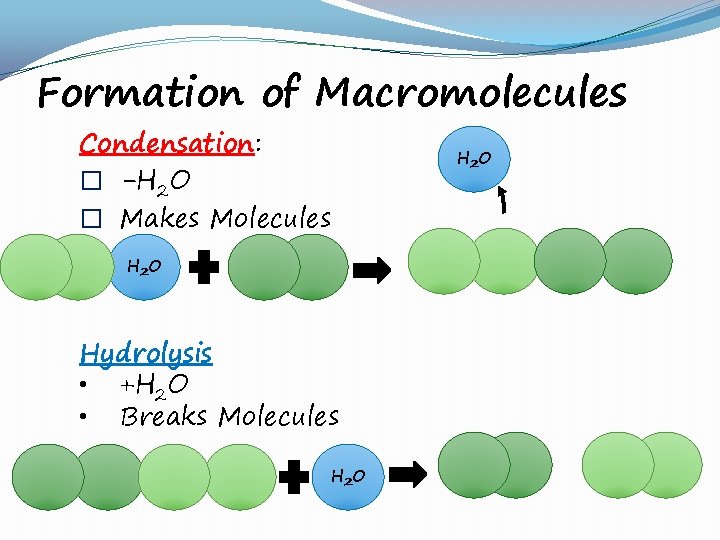
Formation of Macromolecules Condensation: � -H 2 O � Makes Molecules H 2 O Hydrolysis • +H 2 O • Breaks Molecules H 2 O

1. Carbohydrates

Carbohydrate �Ratio for Carbohydrate= 1 C : 2 H : 1 O Functions 1. Easily stored and released Energy 2. Structure (Cellulose-hard part of plants)

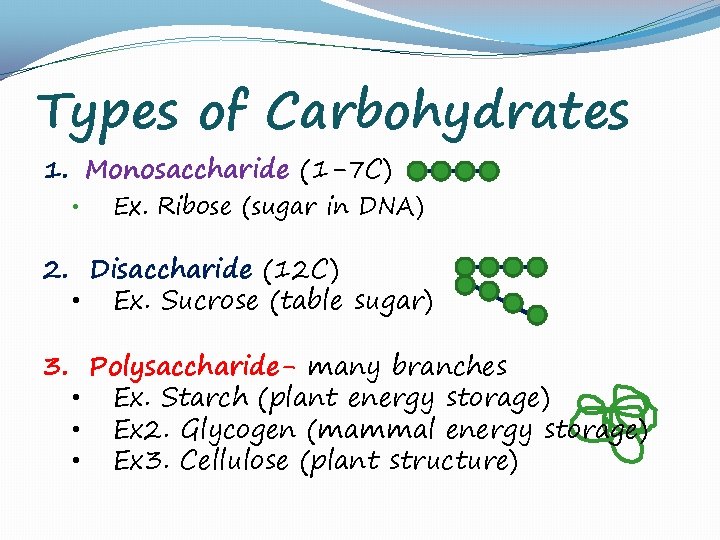
Types of Carbohydrates 1. Monosaccharide (1 -7 C) • Ex. Ribose (sugar in DNA) 2. Disaccharide (12 C) • Ex. Sucrose (table sugar) 3. Polysaccharide- many branches • Ex. Starch (plant energy storage) • Ex 2. Glycogen (mammal energy storage) • Ex 3. Cellulose (plant structure)

2. Lipids (fats)

Lipids �Only made of C & H chains �“Hydrophobic”- Insoluble in Water (due to nonpolar covalent bonds) FUNCTION: �Longterm energy storage �Structure- cell membrane & body surfaces �Thermal insulation

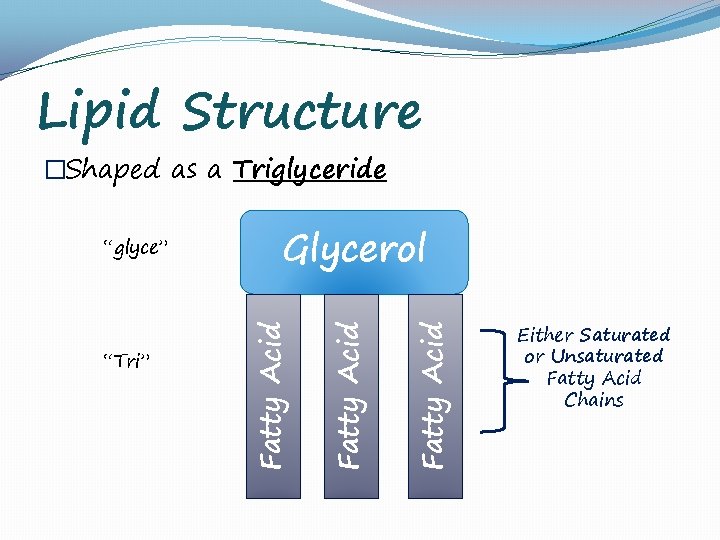
Lipid Structure �Shaped as a Triglyceride Fatty Acid “Tri” Glycerol Fatty Acid “glyce” Either Saturated or Unsaturated Fatty Acid Chains

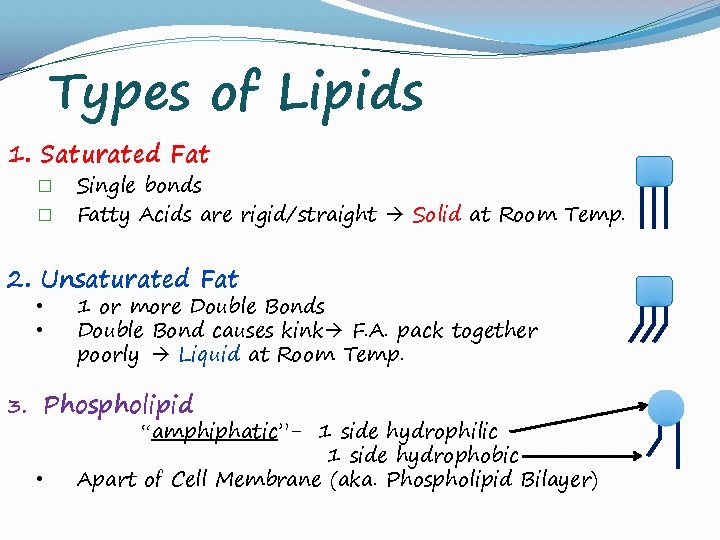
Types of Lipids 1. Saturated Fat � � Single bonds Fatty Acids are rigid/straight Solid at Room Temp. 2. Unsaturated Fat • • 3. 1 or more Double Bonds Double Bond causes kink F. A. pack together poorly Liquid at Room Temp. Phospholipid “amphiphatic”- 1 side hydrophilic • 1 side hydrophobic Apart of Cell Membrane (aka. Phospholipid Bilayer)

Biochemical Reactions

Metabolism �Sum of the total chemical reactions in a system �Uses or provides Energy (E) Types of Energy: 1. Potential- stored energy (fat storage) 2. Kinetic – energy of movements (exercise)

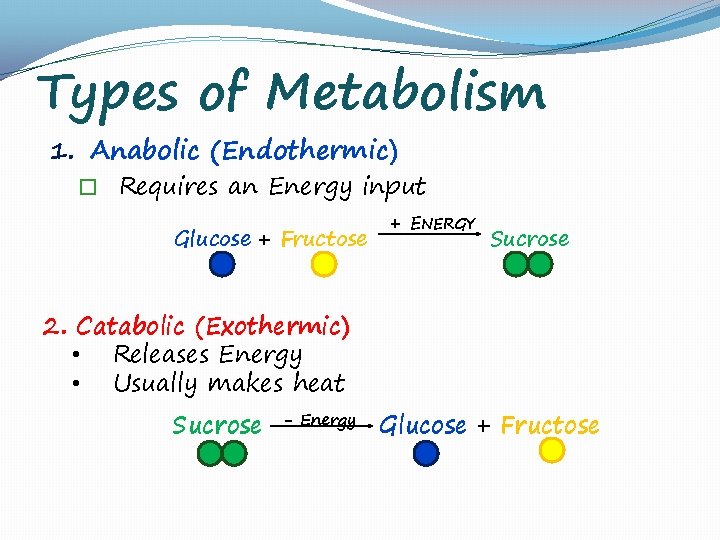
Types of Metabolism 1. Anabolic (Endothermic) � Requires an Energy input Glucose + Fructose + ENERGY Sucrose 2. Catabolic (Exothermic) • Releases Energy • Usually makes heat Sucrose - Energy Glucose + Fructose

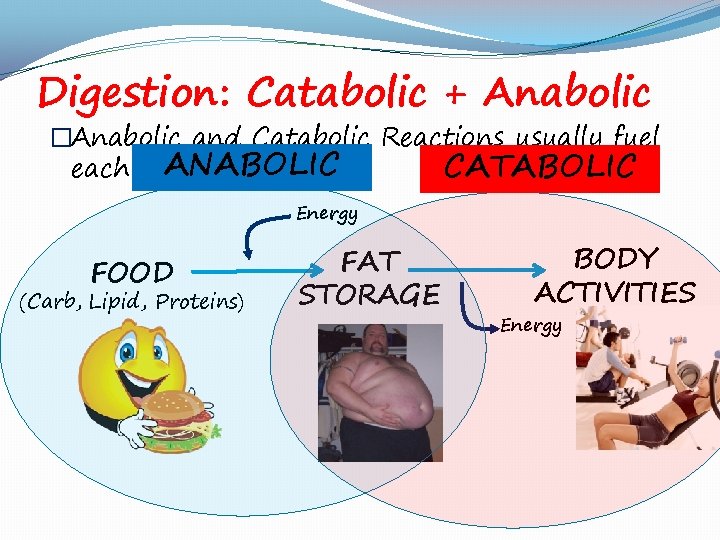
Digestion: Catabolic + Anabolic �Anabolic and Catabolic Reactions usually fuel ANABOLIC CATABOLIC each other Energy FOOD (Carb, Lipid, Proteins) FAT STORAGE BODY ACTIVITIES Energy

Anabolism makes 1 kg of human body, but it requires the catabolism of 10 kg of food

Thermodynamics

Laws of Thermodynamics 1 st Law- Energy is not Created or Destroyed 2 nd Law- Not all Energy is used (some is unusable)

3. Nucleic Acids

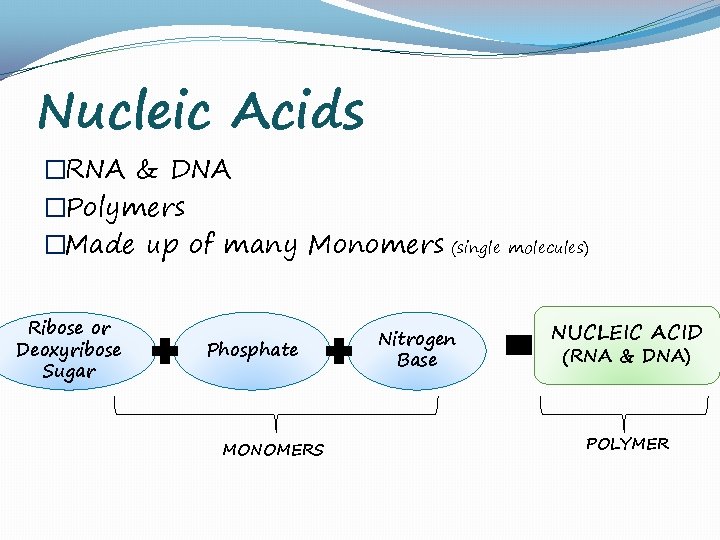
Nucleic Acids �RNA & DNA �Polymers �Made up of many Monomers (single molecules) Ribose or Deoxyribose Sugar Phosphate MONOMERS Nitrogen Base NUCLEIC ACID (RNA & DNA) POLYMER

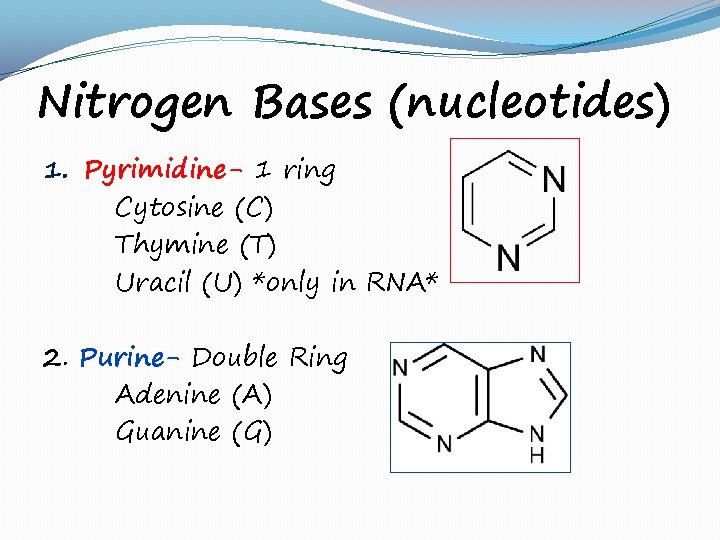
Nitrogen Bases (nucleotides) 1. Pyrimidine- 1 ring Cytosine (C) Thymine (T) Uracil (U) *only in RNA* 2. Purine- Double Ring Adenine (A) Guanine (G)

Types of Nucleotides 1. Oglionucleotides- “Primers”, regulate amount of RNA & DNA 2. Polynucleotides- in RNA & DNA � Largest polymers in the world!

DO NOW List the Purine Nitrogen Bases List the Pyrimidine Nitrogen Base What are the 3 monomers of Nucleic Acids? Which metabolism adds energy to the system? 5. Which metabolism uses energy in the system? 1. 2. 3. 4.

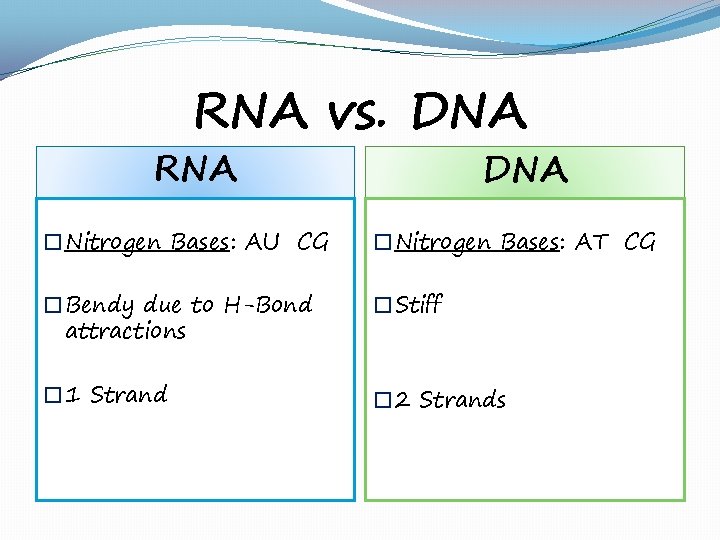
RNA vs. DNA RNA DNA �Nitrogen Bases: AU CG �Nitrogen Bases: AT CG �Bendy due to H-Bond attractions �Stiff � 1 Strand � 2 Strands

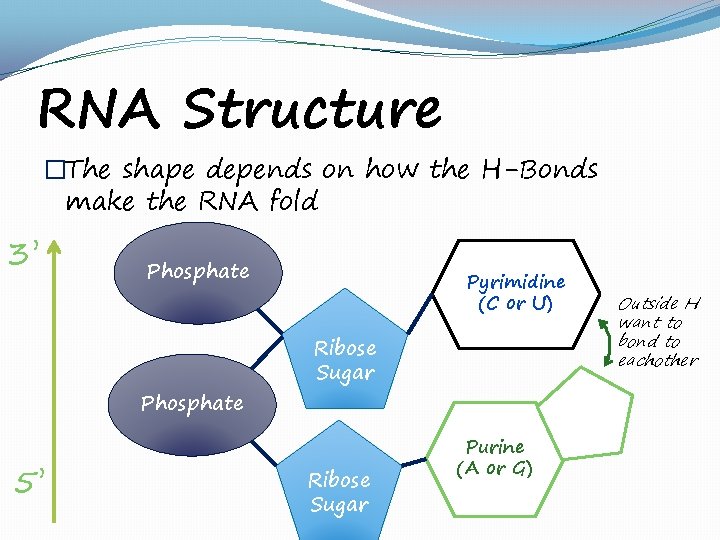
RNA Structure �The shape depends on how the H-Bonds make the RNA fold 3’ Phosphate 5’ Pyrimidine (C or U) Ribose Sugar Purine (A or G) Outside H want to bond to eachother

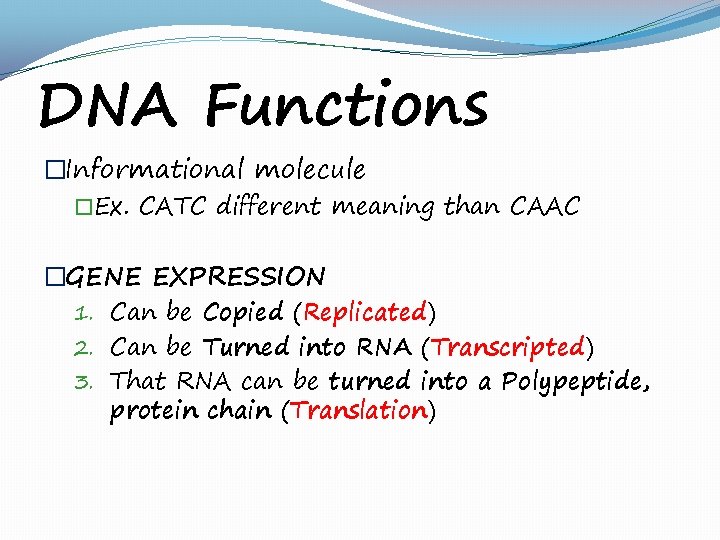
DNA Functions �Informational molecule �Ex. CATC different meaning than CAAC �GENE EXPRESSION 1. Can be Copied (Replicated) 2. Can be Turned into RNA (Transcripted) 3. That RNA can be turned into a Polypeptide, protein chain (Translation)

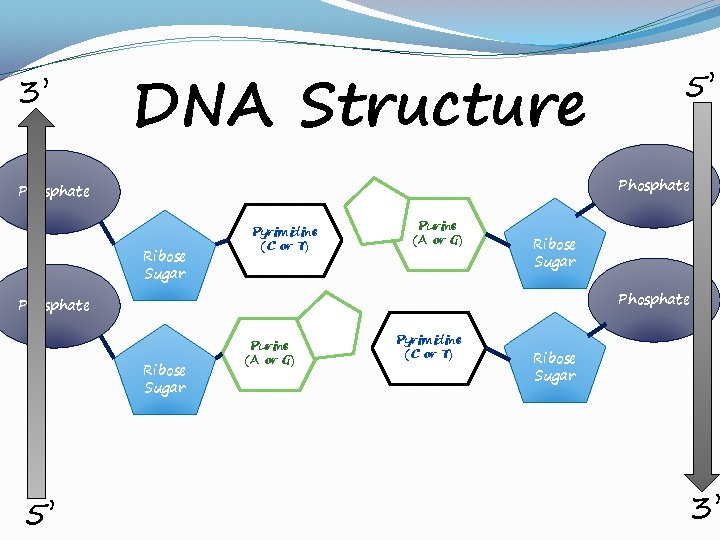
3’ DNA Structure Phosphate Ribose Sugar Pyrimidine (C or T) Purine (A or G) Ribose Sugar Phosphate Ribose Sugar 5’ 5’ Purine (A or G) Pyrimidine (C or T) Ribose Sugar 3’

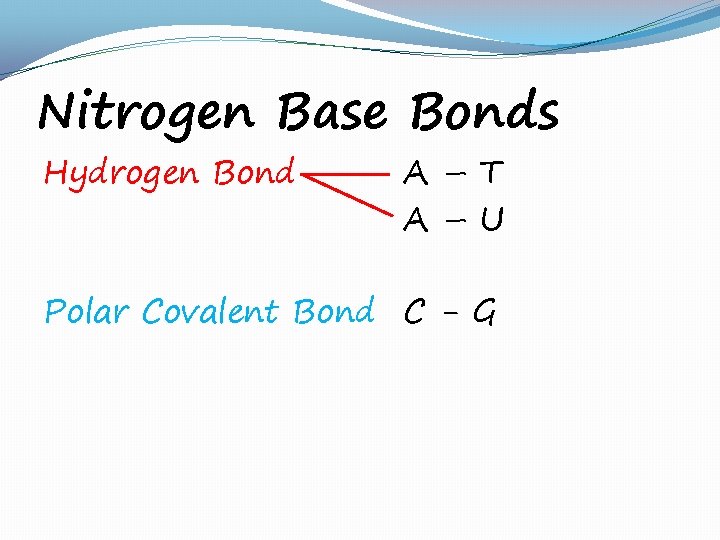
Nitrogen Base Bonds Hydrogen Bond A–T A–U Polar Covalent Bond C - G

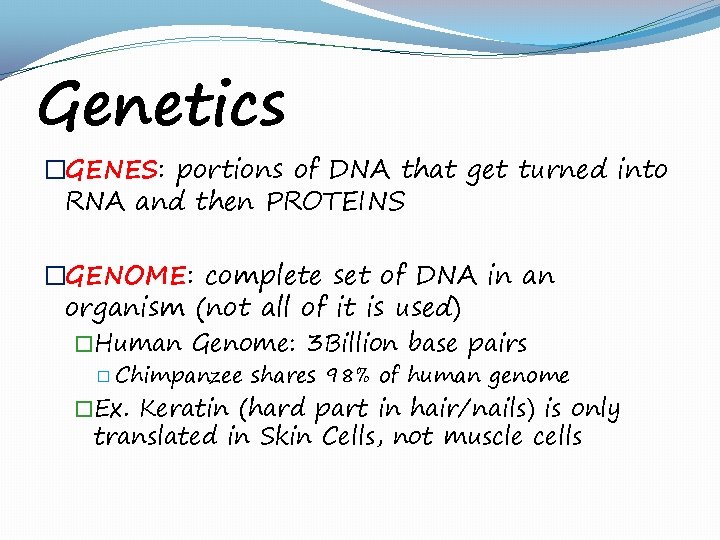
Genetics �GENES: portions of DNA that get turned into RNA and then PROTEINS �GENOME: complete set of DNA in an organism (not all of it is used) �Human Genome: 3 Billion base pairs � Chimpanzee shares 98% of human genome �Ex. Keratin (hard part in hair/nails) is only translated in Skin Cells, not muscle cells

4) PROTEINS

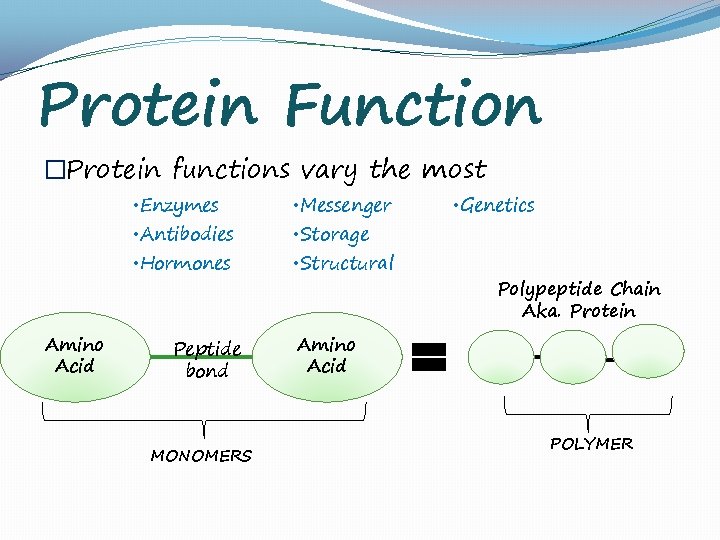
Protein Function �Protein functions vary the most • Enzymes • Messenger • Hormones • Structural • Antibodies Amino Acid Peptide bond MONOMERS • Storage • Genetics Polypeptide Chain Aka. Protein Amino Acid POLYMER

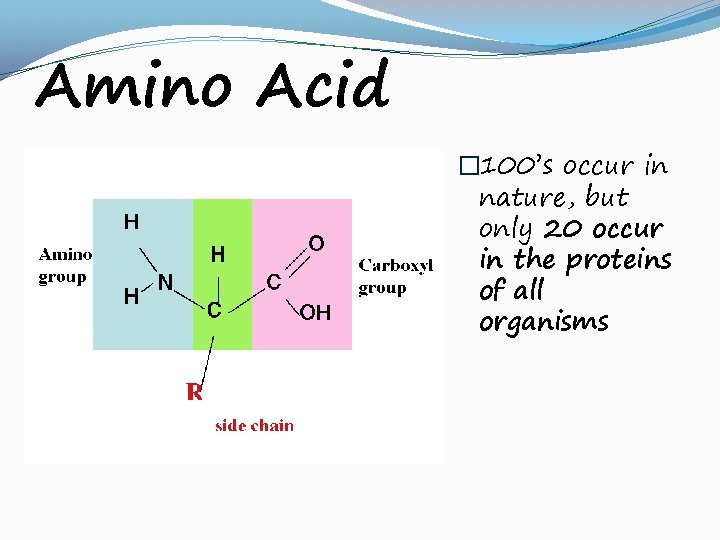
Amino Acid � 100’s occur in nature, but only 20 occur in the proteins of all organisms

Protein Structure

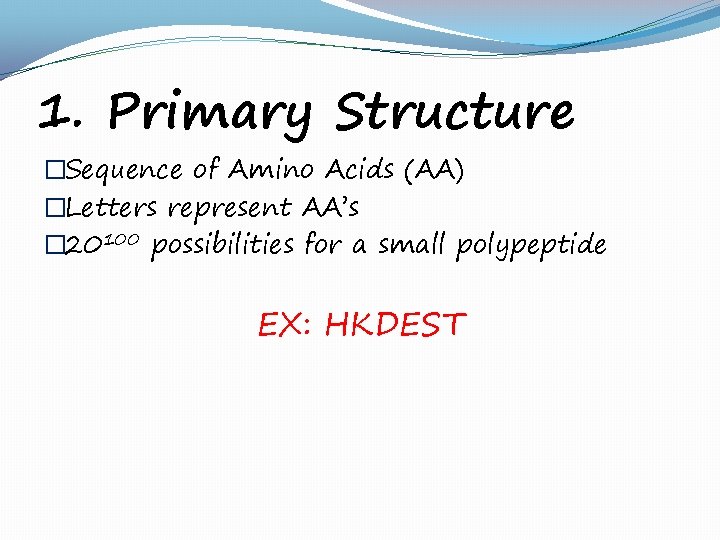
1. Primary Structure �Sequence of Amino Acids (AA) �Letters represent AA’s � 20100 possibilities for a small polypeptide EX: HKDEST

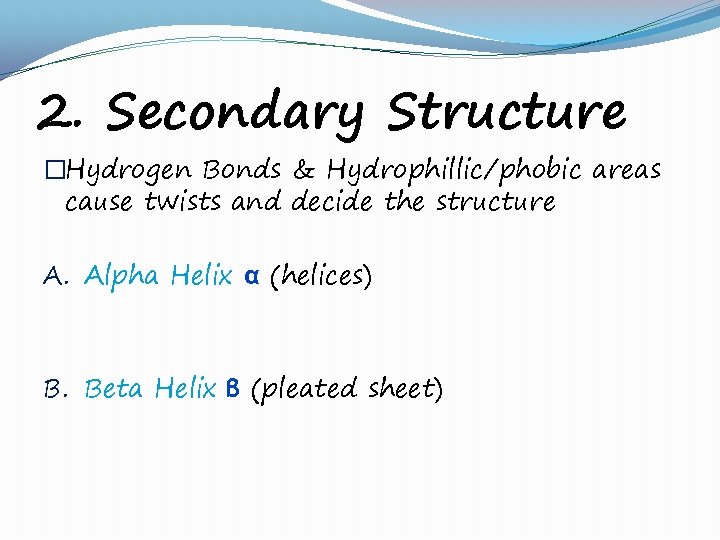
2. Secondary Structure �Hydrogen Bonds & Hydrophillic/phobic areas cause twists and decide the structure A. Alpha Helix α (helices) B. Beta Helix β (pleated sheet)

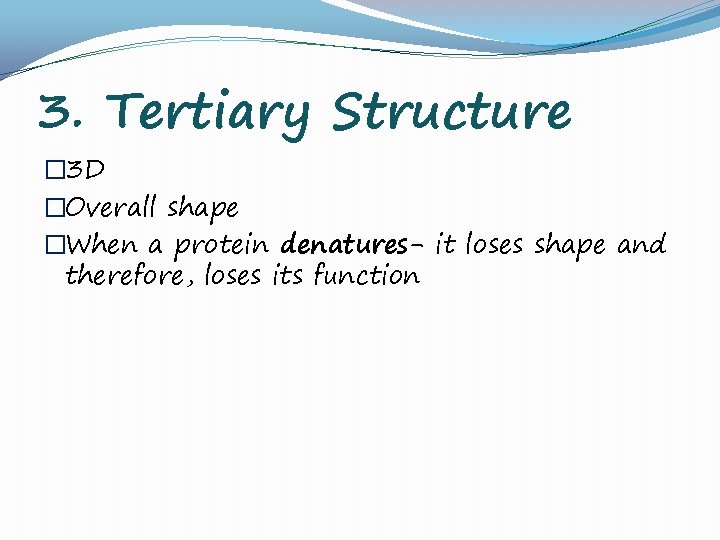
3. Tertiary Structure � 3 D �Overall shape �When a protein denatures- it loses shape and therefore, loses its function

4. Quaternary Structure � 2+ polypeptides combined �Ex. Spiderweb proteins are so strong because numerous interlocking B helices

Denaturing �Because proteins are held together by weaker forces, they are easily destroyed �DENATURE: destroys protein structure �Can be irreversible � Heat � p. H � Too much polar or nonpolar