DO NOW Pick up handout Get out Concept





- Slides: 20

DO NOW • Pick up handout. • Get out Concept Review handout.

Types of Chemical Reactions • There are five basic types of chemical reactions. • Not all reactions will take these five forms. • Other classes of reactions can include. • net ionic • oxidation-reduction • neutralization

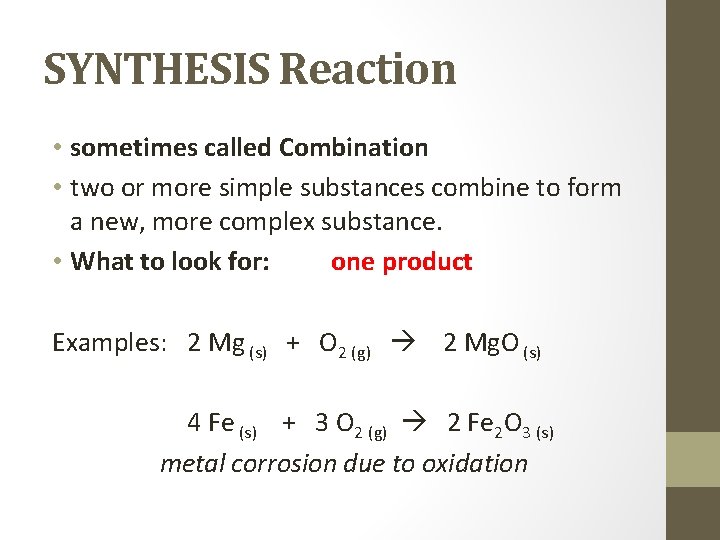
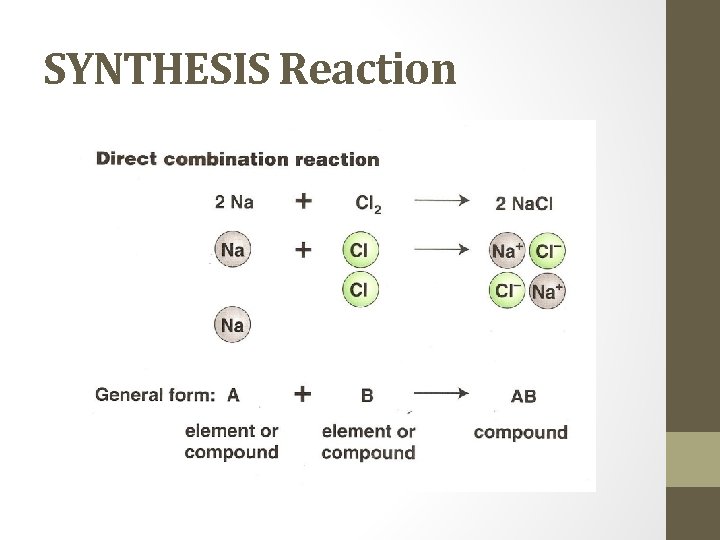
SYNTHESIS Reaction • sometimes called Combination • two or more simple substances combine to form a new, more complex substance. • What to look for: one product Examples: 2 Mg (s) + O 2 (g) 2 Mg. O (s) 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) metal corrosion due to oxidation

SYNTHESIS Reaction

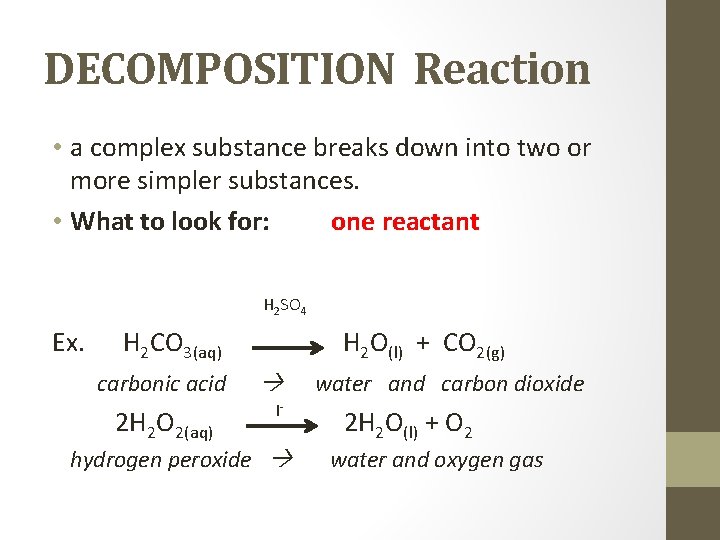
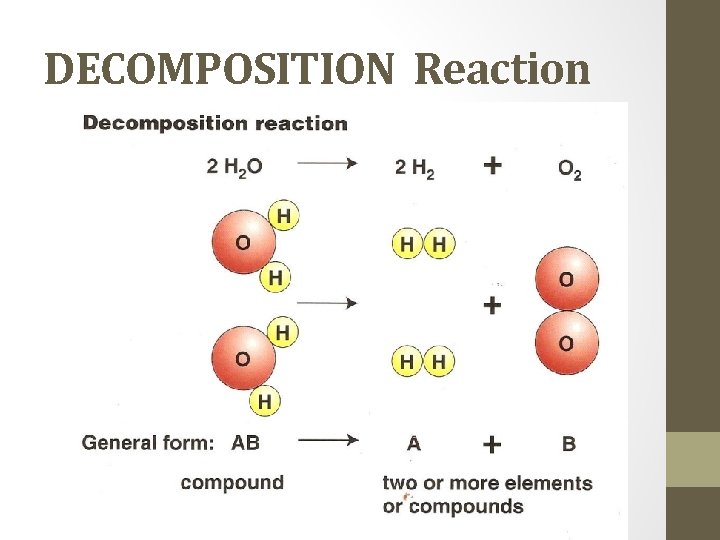
DECOMPOSITION Reaction • a complex substance breaks down into two or more simpler substances. • What to look for: one reactant H 2 SO 4 Ex. H 2 CO 3(aq) H 2 O(l) + CO 2(g) carbonic acid 2 H 2 O 2(aq) I- hydrogen peroxide water and carbon dioxide 2 H 2 O(l) + O 2 water and oxygen gas

DECOMPOSITION Reaction

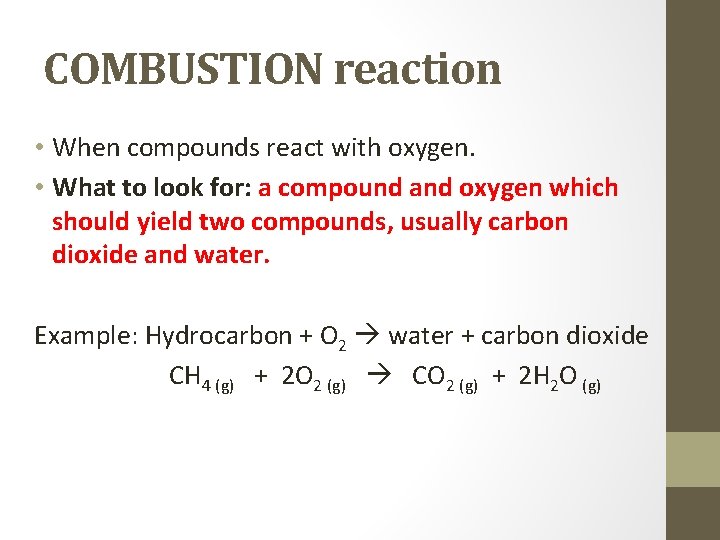
COMBUSTION reaction • When compounds react with oxygen. • What to look for: a compound and oxygen which should yield two compounds, usually carbon dioxide and water. Example: Hydrocarbon + O 2 water + carbon dioxide CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g)


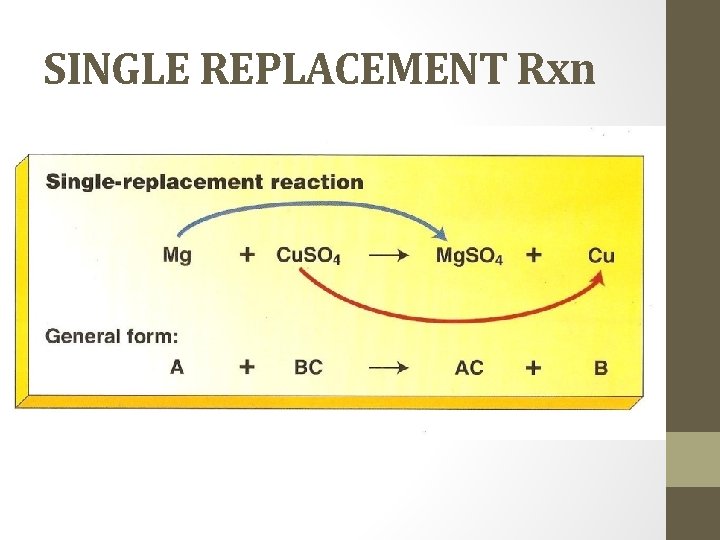
SINGLE REPLACEMENT Rxn • Also called single displacement reaction. • one element replaces another element in a compound. • Use the activity series of metals to determine if the metal can be replaced or not. • What to look for: uncombined elements on both sides of the arrow

SINGLE REPLACEMENT Rxn

ACTIVITY SERIES


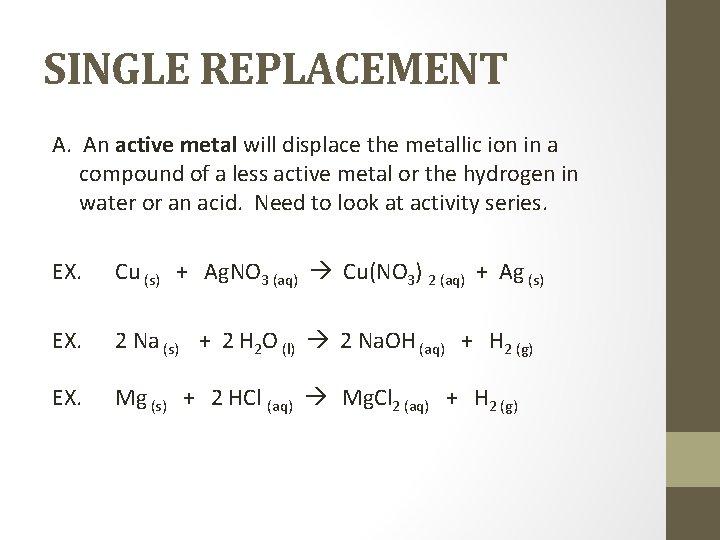
SINGLE REPLACEMENT A. An active metal will displace the metallic ion in a compound of a less active metal or the hydrogen in water or an acid. Need to look at activity series. EX. Cu (s) + Ag. NO 3 (aq) Cu(NO 3) 2 (aq) + Ag (s) EX. Mg (s) + 2 HCl (aq) Mg. Cl 2 (aq) + H 2 (g) 2 Na (s) + 2 H 2 O (l) 2 Na. OH (aq) + H 2 (g)

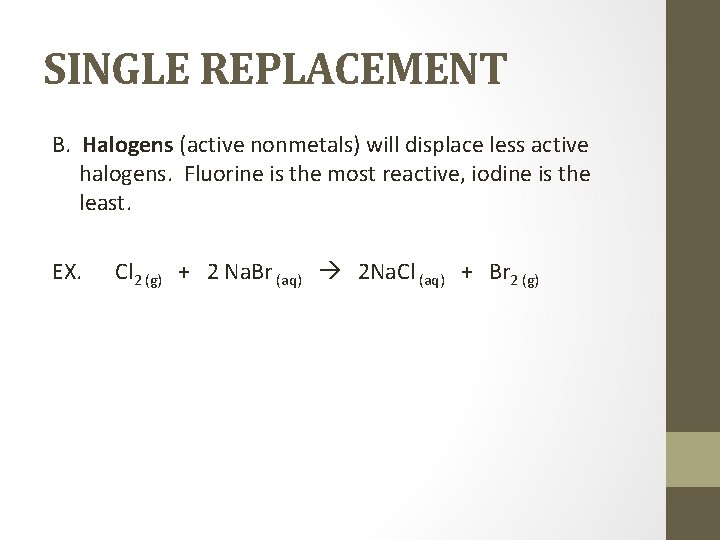
SINGLE REPLACEMENT B. Halogens (active nonmetals) will displace less active halogens. Fluorine is the most reactive, iodine is the least. EX. Cl 2 (g) + 2 Na. Br (aq) 2 Na. Cl (aq) + Br 2 (g)


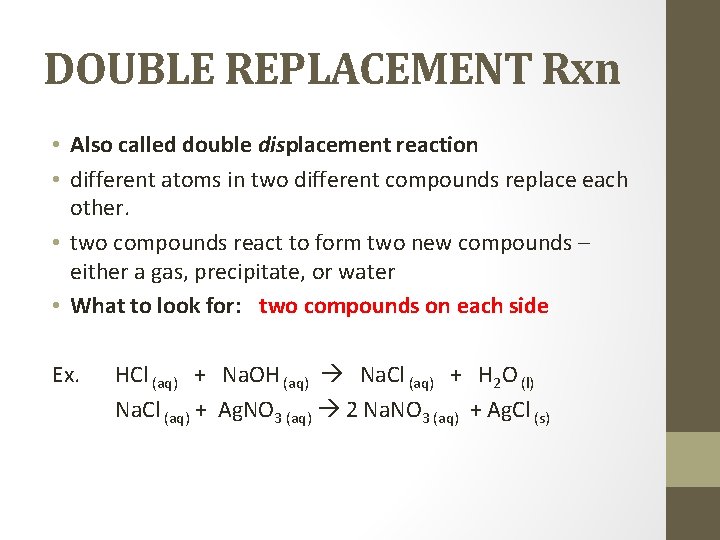
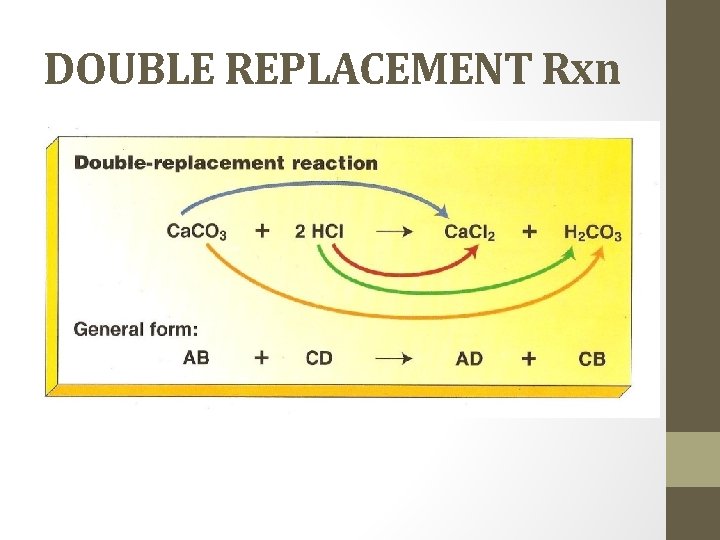
DOUBLE REPLACEMENT Rxn • Also called double displacement reaction • different atoms in two different compounds replace each other. • two compounds react to form two new compounds – either a gas, precipitate, or water • What to look for: two compounds on each side Ex. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) Na. Cl (aq) + Ag. NO 3 (aq) 2 Na. NO 3 (aq) + Ag. Cl (s)

DOUBLE REPLACEMENT Rxn

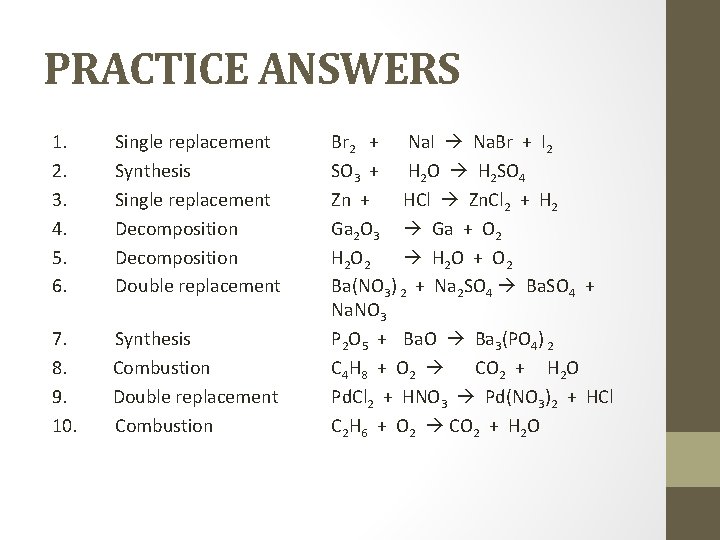
PRACTICE 1. Br 2 + Na. I Na. Br + I 2 2. SO 3 + H 2 O H 2 SO 4 3. Zn + HCl Zn. Cl 2 + H 2 4. Ga 2 O 3 Ga + O 2 5. H 2 O 2 H 2 O + O 2 6. Ba(NO 3) 2 + Na 2 SO 4 Ba. SO 4 + Na. NO 3 7. P 2 O 5 + Ba. O Ba 3(PO 4) 2 8. C 4 H 8 + O 2 CO 2 + H 2 O 9. Pd. Cl 2 + HNO 3 Pd(NO 3)2 + HCl 10. C 2 H 6 + O 2 CO 2 + H 2 O

PRACTICE ANSWERS 1. 2. 3. 4. 5. 6. Single replacement Synthesis Single replacement Decomposition Double replacement 7. Synthesis 8. Combustion 9. Double replacement 10. Combustion Br 2 + Na. I Na. Br + I 2 SO 3 + H 2 O H 2 SO 4 Zn + HCl Zn. Cl 2 + H 2 Ga 2 O 3 Ga + O 2 H 2 O + O 2 Ba(NO 3) 2 + Na 2 SO 4 Ba. SO 4 + Na. NO 3 P 2 O 5 + Ba. O Ba 3(PO 4) 2 C 4 H 8 + O 2 CO 2 + H 2 O Pd. Cl 2 + HNO 3 Pd(NO 3)2 + HCl C 2 H 6 + O 2 CO 2 + H 2 O

TO DO • Balancing and Classifying Chemical Equations – due tomorrow.