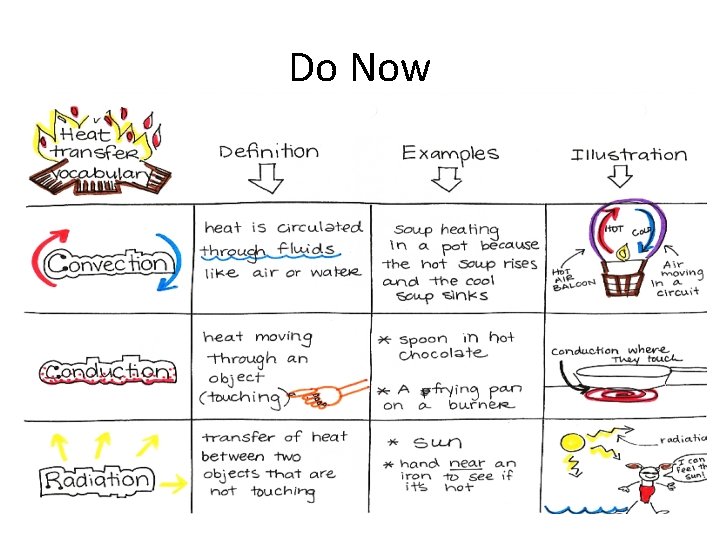
Do Now How could we make the room





























- Slides: 49

Do Now • How could we make the room warmer/cooler? • Is the amount of heat to raise the temperature of a small room by 2 degrees the same as it is to raise the temperature of a large room by 2 degrees? Why?

Do Now • We can make the room warmer by adding heat or cooler by removing heat • No, the temperature is not the same, the larger room would require more heat to raise the temperature.

Monday Shout Outs • High Scores on the test! – – – Marquell Brianna A. Cedrick Latavia Juliette Lance Leoriandy Kyle Cynthia Dillon Naje

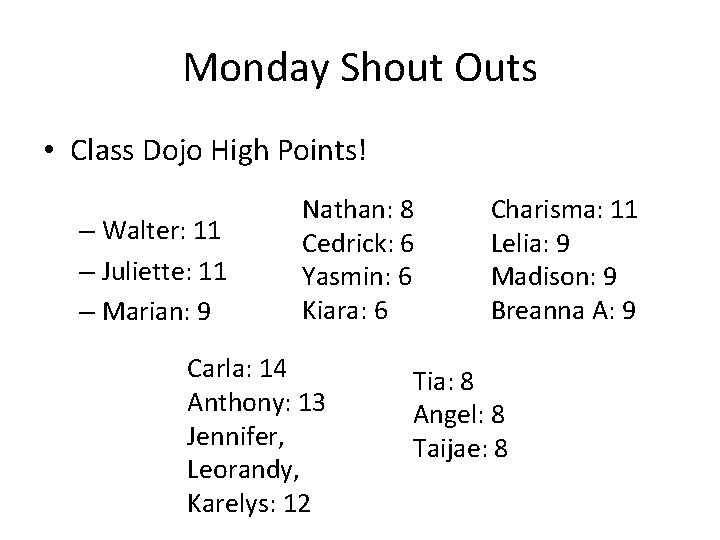
Monday Shout Outs • Class Dojo High Points! – Walter: 11 – Juliette: 11 – Marian: 9 Nathan: 8 Cedrick: 6 Yasmin: 6 Kiara: 6 Carla: 14 Anthony: 13 Jennifer, Leorandy, Karelys: 12 Charisma: 11 Lelia: 9 Madison: 9 Breanna A: 9 Tia: 8 Angel: 8 Taijae: 8

Heat Objective • Students will learn to identify conditions under which thermal energy tends to flow

Heat Essential Question • How is thermal energy transferred? • What causes an object to change temperature? • Explain the difference between heat and temperature?

Heat Radiation Conduction Specific heat Convection

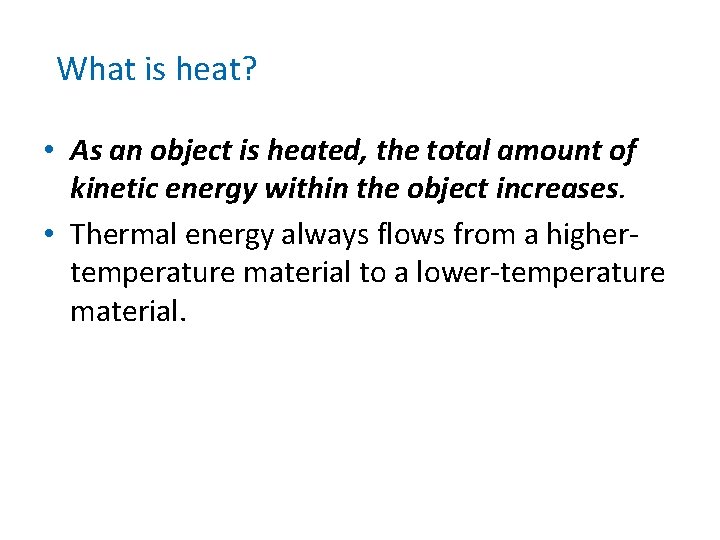
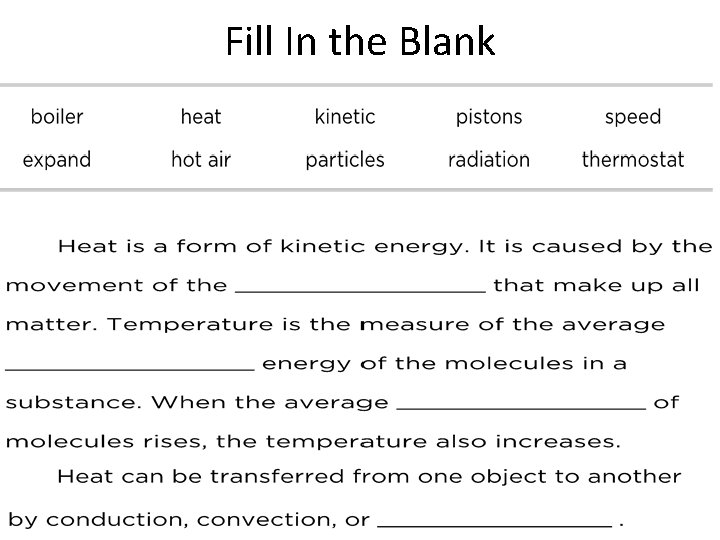
What is heat? • Heat the flow of thermal energy from warmer to cooler objects. • Thermal energy: energy due to particle movement

What is heat? • As an object is heated, the total amount of kinetic energy within the object increases. • Thermal energy always flows from a highertemperature material to a lower-temperature material.

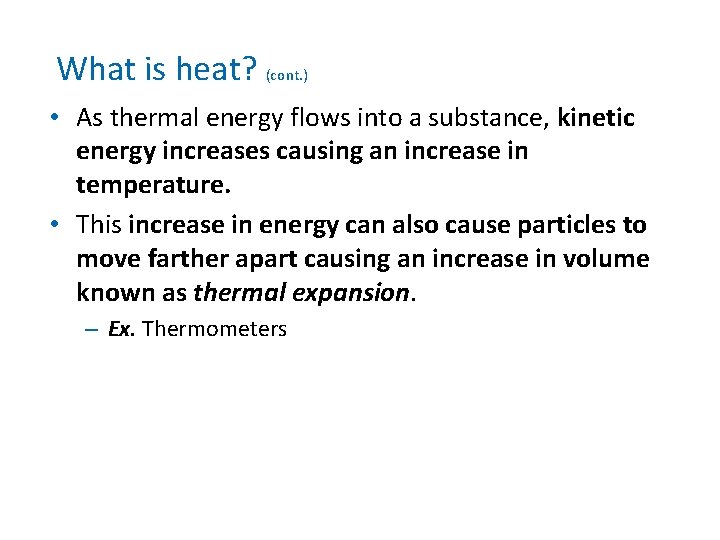
What is heat? (cont. ) • As thermal energy flows into a substance, kinetic energy increases causing an increase in temperature. • This increase in energy can also cause particles to move farther apart causing an increase in volume known as thermal expansion. – Ex. Thermometers

What is heat? (cont. ) • Total amount of thermal energy depends on temperature and mass. • Think about it – Does high temperature mean high amount of heat?

Think about it • Does high temperature mean high amount of heat? – High temperature does not necessarily mean a great amount of heat. – A great amount of heat does not necessarily mean high temperature. – Boiling water has a high temperature, the ocean has a much lower temperature, but an ocean has more heat energy because of the mass

Think about it • At the Big Island of Hawaii, hot, molten lava flows into the sea. The temperature of the lava is much higher than that of the seawater. • What happens to the water when the lava reaches it?

Think about it • What happens to the water when the lava reaches it? – As the lava reaches the water, heat flows from the lava to the water which becomes hotter. The particles in the water speed up and spread out until some turns some into water vapor.

Practice • Heat from Friction – Form a hypothesis – Read the procedure – Record data – Intemperate data and infer

Review • Heat is the flow of thermal energy from warmer to cooler objects • Increase in energy can also cause particles to move farther apart causing an increase in volume known as thermal expansion.

Do Now • In what direction does heat flow? • What causes an object to increase temperature?

Do Now • Heat flows from one substance to another from warm to cool • An increase in thermal energy causes an object to change temperature

Heat Objective • Students will learn to compare how thermal energy can be transferred by conduction, convection and radiation

Heat Essential Question • Explain the difference between condition, convection and radiation • What are every day examples of conduction, convection, and radiation? • How is specific heat related to temperature? • Which type of heat transfer do you come in contact with the most?

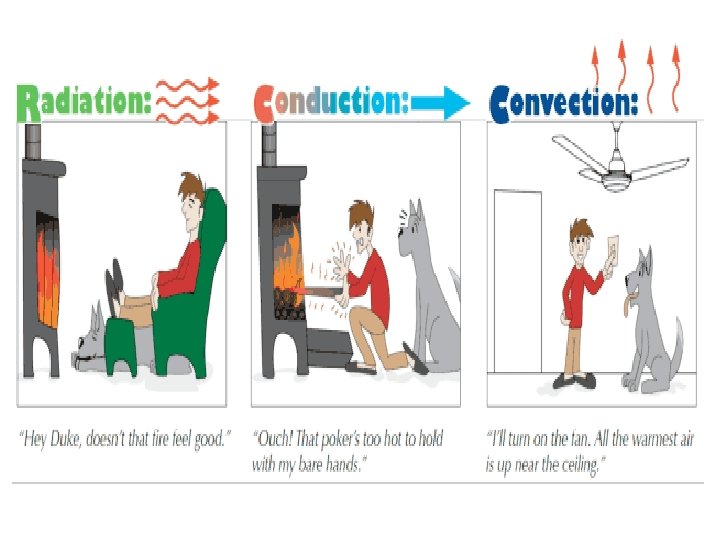
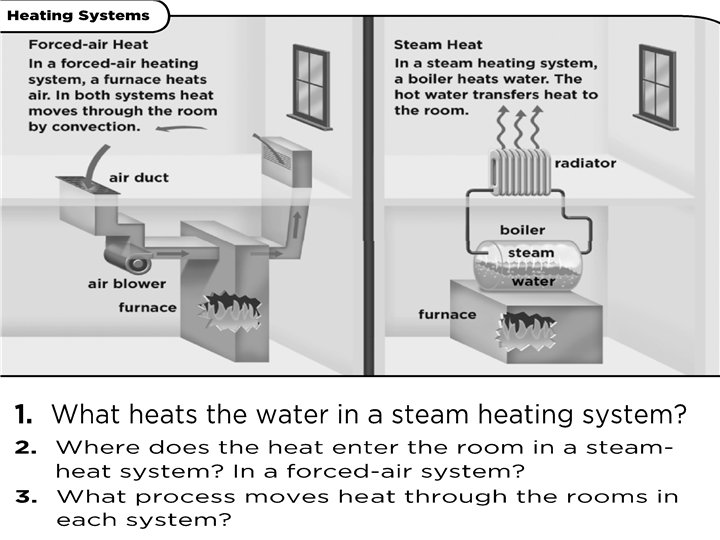
How does heat travel? • Thermal energy is transferred in three ways. – By conduction – By convection – By radiation

How does heat travel?

How does heat travel?

How does heat travel?

How does heat travel?

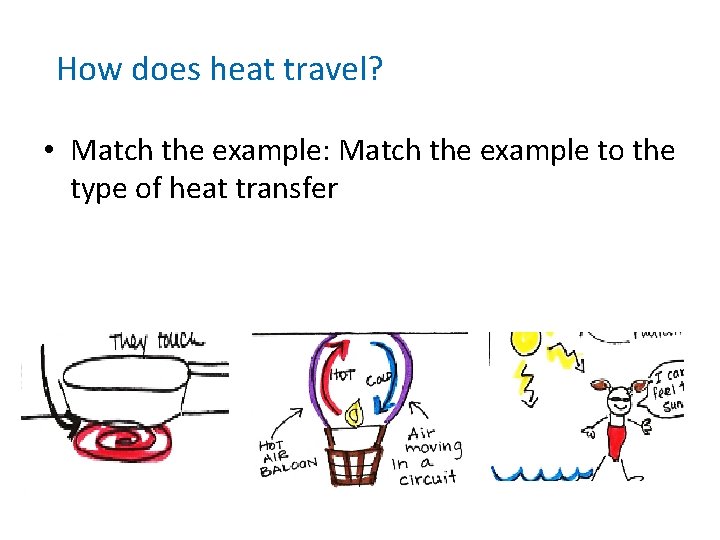
How does heat travel? • Match the example: Match the example to the type of heat transfer


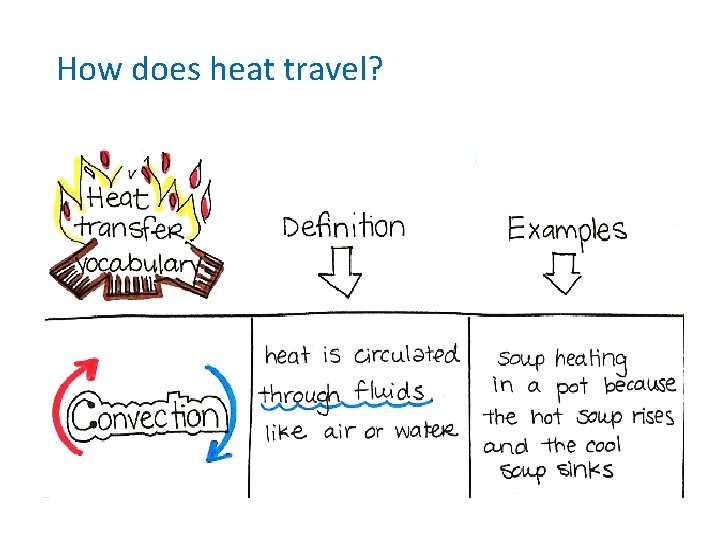
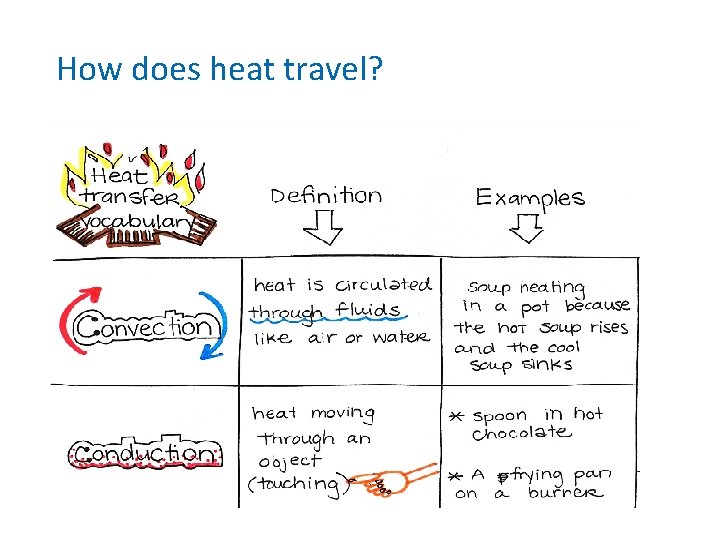
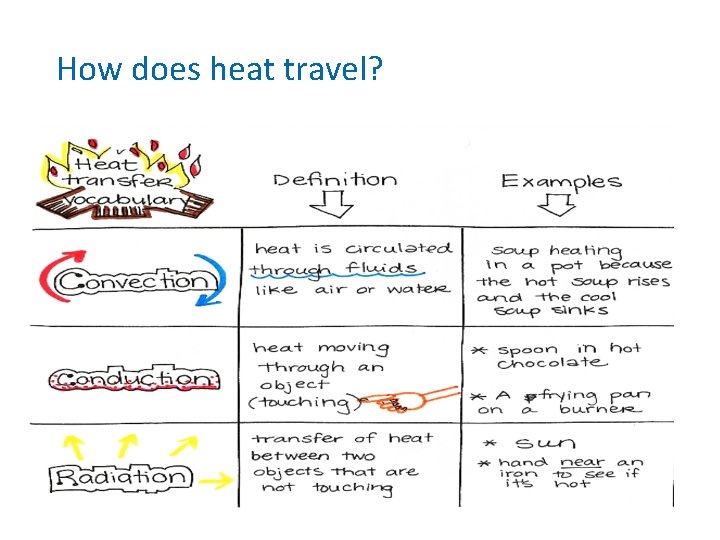
How does heat travel? (cont. ) • Conduction the movement of energy, such as heat or electricity, through direct contact. – Occurs in solids, liquids and gases. In your group: List 2 examples of conduction

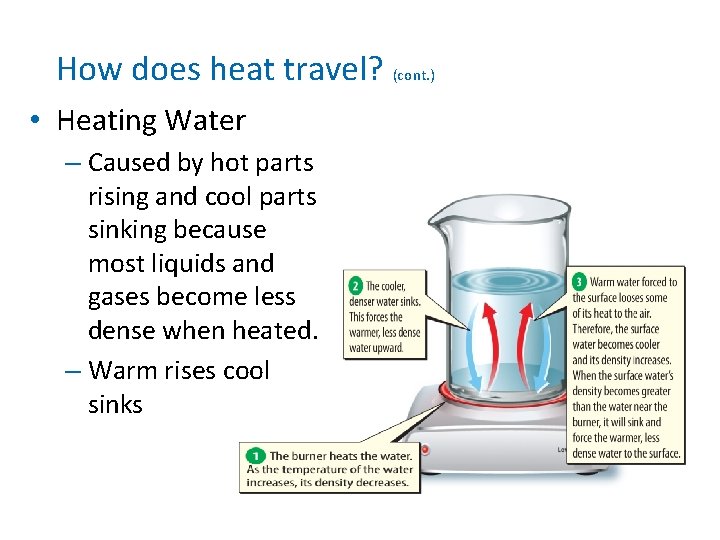
How does heat travel? (cont. ) • Convection the transfer of energy by the flow of a liquid or a gas. • Why is heating water an example of convection?

How does heat travel? (cont. ) • Heating Water – Caused by hot parts rising and cool parts sinking because most liquids and gases become less dense when heated. – Warm rises cool sinks

How does heat travel? (cont. ) • Radiation the transfer of thermal energy through electromagnetic rays. – No medium is necessary because electromagnetic rays can travel through empty space. – Ex. Sun heating Earth, toasters, ovens.

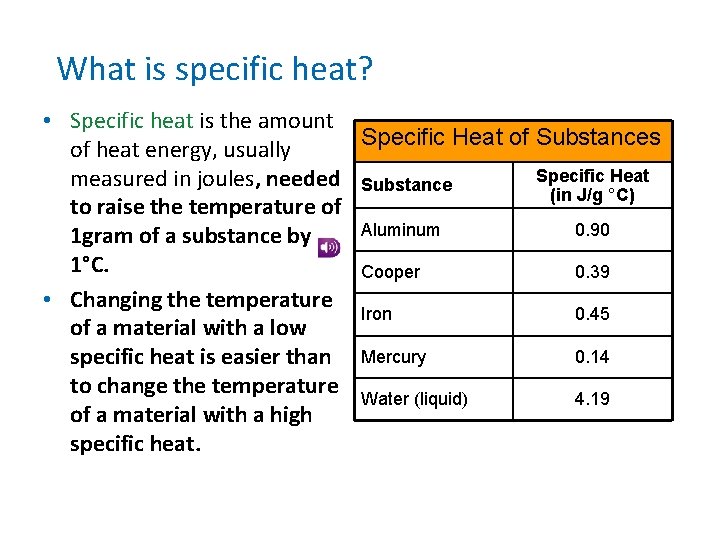
What is specific heat? • Specific heat is the amount Specific Heat of Substances of heat energy, usually Specific Heat measured in joules, needed Substance (in J/g °C) to raise the temperature of Aluminum 0. 90 1 gram of a substance by 1°C. Cooper 0. 39 • Changing the temperature Iron 0. 45 of a material with a low 0. 14 specific heat is easier than Mercury to change the temperature Water (liquid) 4. 19 of a material with a high specific heat.

What is specific heat? • How is specific heat related to temperature? • Specific heat is the amount of energy needed to raise the temperature of 1 gram of a substance by 1 degree Celsius

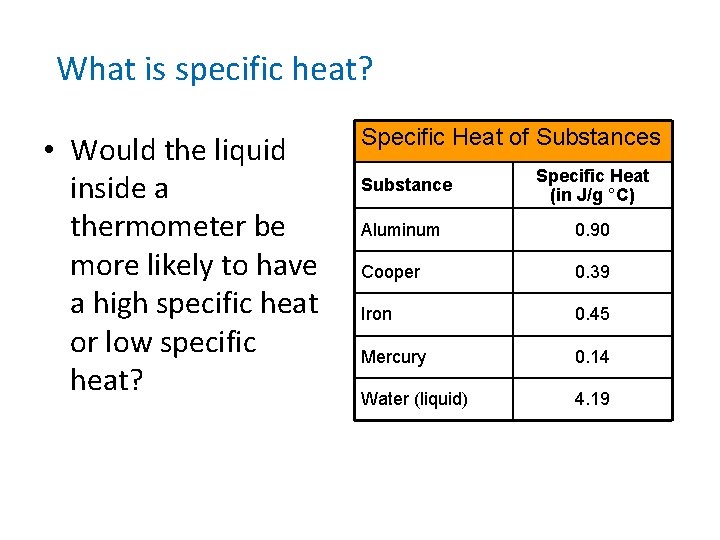
What is specific heat? • Would the liquid inside a thermometer be more likely to have a high specific heat or low specific heat? Specific Heat of Substances Substance Specific Heat (in J/g °C) Aluminum 0. 90 Cooper 0. 39 Iron 0. 45 Mercury 0. 14 Water (liquid) 4. 19




Exit Quiz

Do Now • What is the difference between conduction, convection, radiation?

Do Now

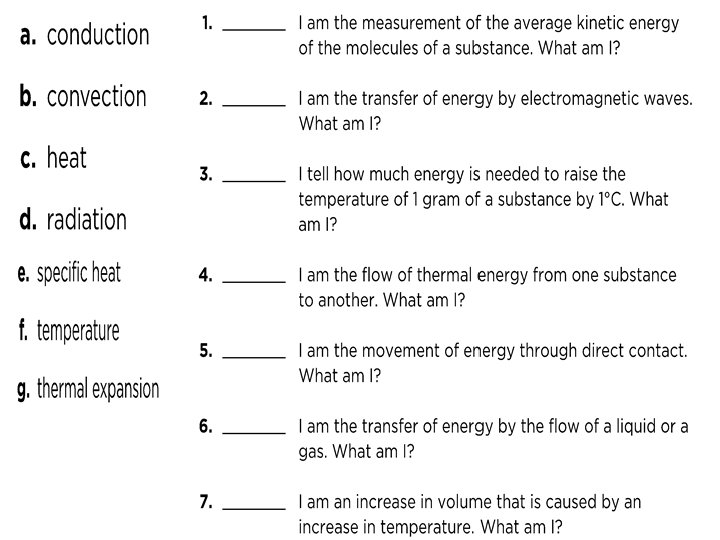
Fill In the Blank

e. Review Heat • Be prepared to write and discuss upcoming questions

What’s Next • Silently put everything away • There should be nothing on your desk

Matching Game • Be very careful when opening the bag, there are many small matching pieces • The front and back sheet has the directions for each section • Use the folded page for puzzle piece reference • For the journal activity, you do not need to write it out, discuss with your group, be prepared to share to the class

Heat Transfer Review

Closing Activity Worksheet • Without using your textbook or notes compete the paragraph • Tests on heat transfer tomorrow!

Do Now • A pot of tea and a cup of tea have the same temperature. However, thermal energy of the pot of tea is higher than thermal energy of the cup of tea. How is this possible?

Do Now • Thermal energy, but not temperature, depends on how much of a substance is present. The water molecules in the pot and cup have the same average kinetic energy. Therefore, the water temperature is the same. However, The pot has more water molecules that the cup. There fore the pot of tea has more thermal energy

Heat Transfer Test • Voices at a level 0!