Do Now Draw a Bohr Diagram of Neon
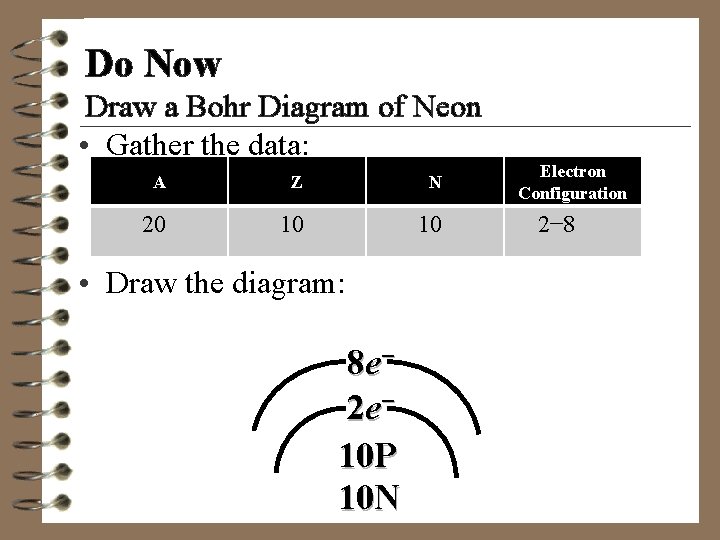
Do Now Draw a Bohr Diagram of Neon • Gather the data: A Z N 20 10 10 • Draw the diagram: 8 e− 2 e− 10 P 10 N Electron Configuration 2− 8

A. K. A. Lewis Dot Structures
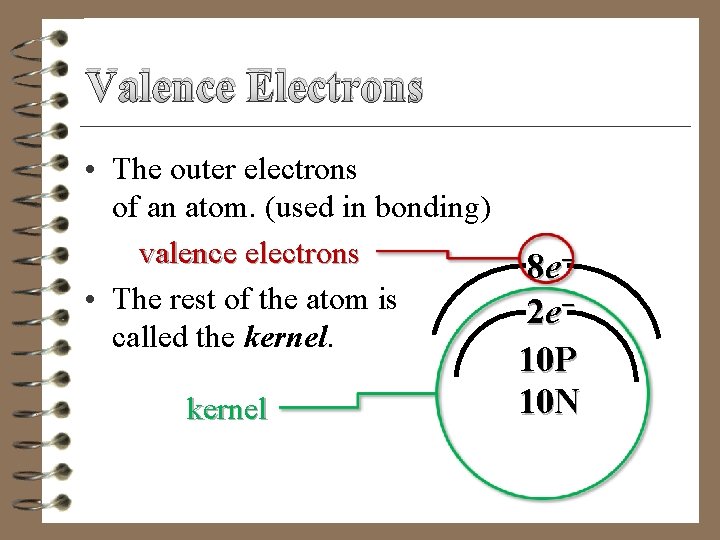
Valence Electrons • The outer electrons of an atom. (used in bonding) valence electrons • The rest of the atom is called the kernel 8 e− 2 e− 10 P 10 N
Electron Dot Diagram • The kernel of an atom, in an electron dot diagram, is represented by the element’s symbol. • The valence electrons are represented by dots. o The location of these dots are four sides of the symbol o Up to 2 electrons can be placed in each location.
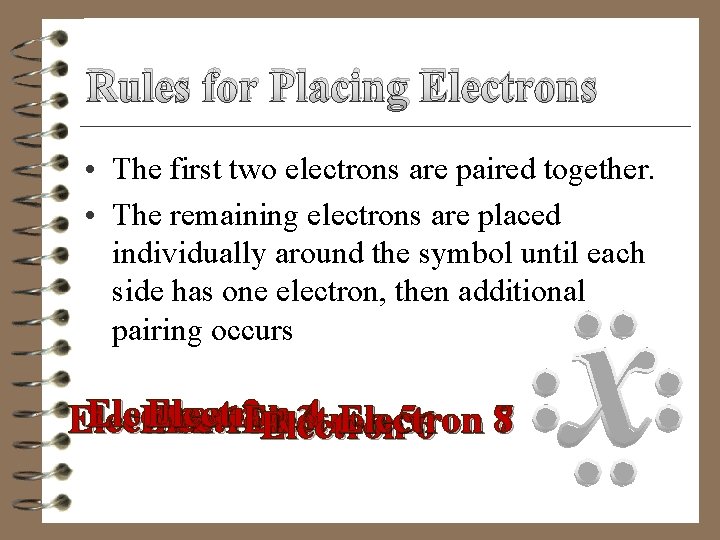
Rules for Placing Electrons • The first two electrons are paired together. • The remaining electrons are placed individually around the symbol until each side has one electron, then additional pairing occurs Electron 2 Electron 1 Electron 34 Electron 56 87
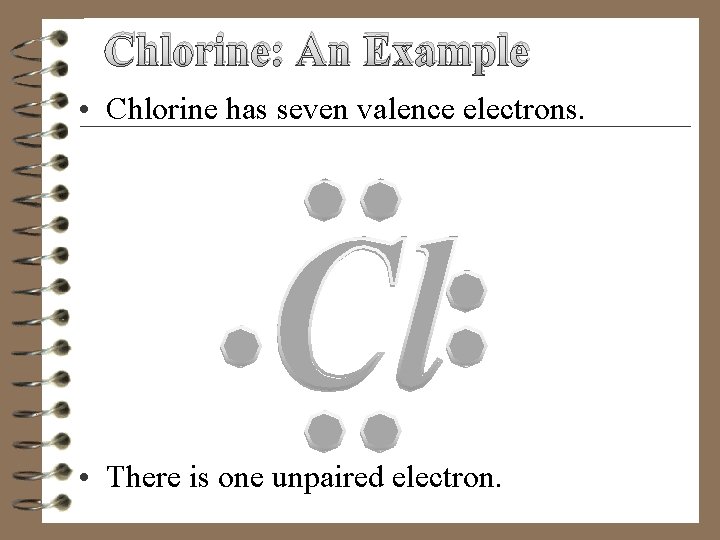
Chlorine: An Example • Chlorine has seven valence electrons. • There is one unpaired electron.

Ions • An atom that does not have a neutral charge • Negative Ions • Have extra electrons giving the atom an overall negative charge Cl. Has one extra electron • Positive Ions • Have fewer electrons giving the atom an overall positive charge Mg 2+ Missing 2 electrons
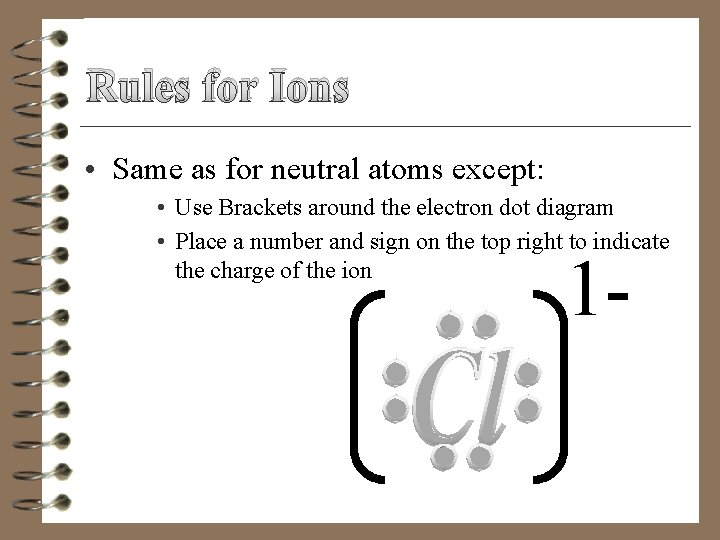
Rules for Ions • Same as for neutral atoms except: • Use Brackets around the electron dot diagram • Place a number and sign on the top right to indicate the charge of the ion 1 -
Practice • Draw the electron dot diagram for Rb Rb+
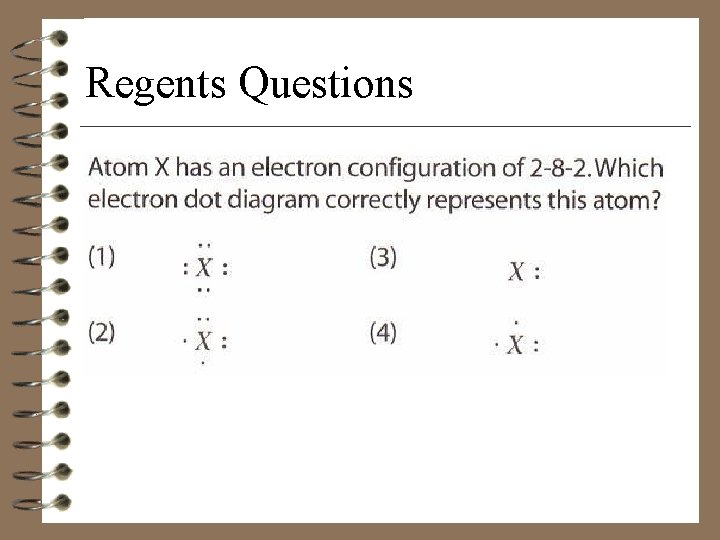
Regents Questions
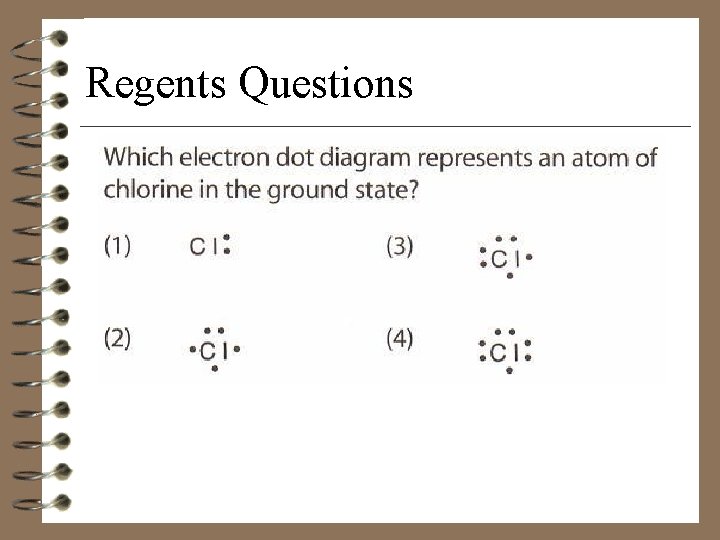
Regents Questions
Bellwork • Draw both the Bohr diagram and the Electron dot diagram for a Calcium ion with a charge of 2+
- Slides: 12