Do Now AEROBIC RESPIRATION Early Human Technology What

Do Now: AEROBIC RESPIRATION

Early Human Technology � What earliest technology makes homo unique among animals? Pan troglodyte Homo neanderthalis Ardipithicus (4. 4 MYA)

Not Tools… Chimpanzees use those too

FIRE!!! � Archaeological sites in Asia and Europe indicate the first controlled use of fire occurred about 1, 500, 000 years ago. � The location of these ancient campfires coincides with the discovery of Homo erectus remains.
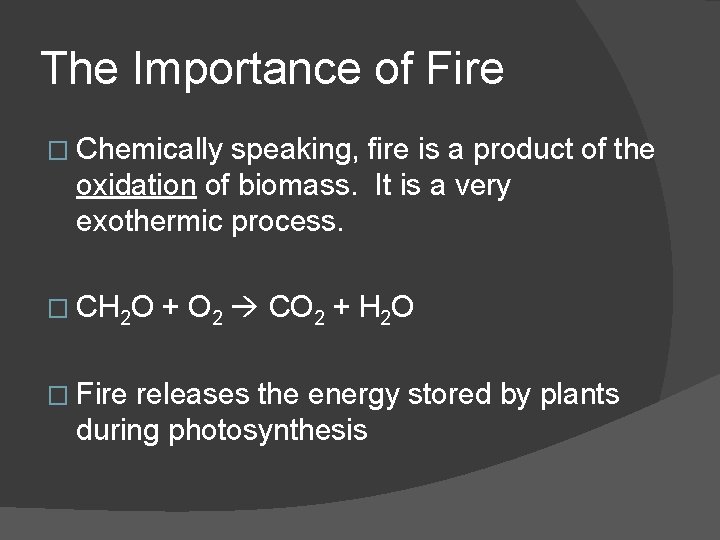
The Importance of Fire � Chemically speaking, fire is a product of the oxidation of biomass. It is a very exothermic process. � CH 2 O � Fire + O 2 CO 2 + H 2 O releases the energy stored by plants during photosynthesis

Fire & Cellular Respiration – Same Thing!!! � The examples shown have different amounts of energy stored in them. Rank them from least energy to greatest

What Fire Represents � The discovery of fire represented an increase in the amount of energy available to humans. � We have used this energy to increase our numbers and colonize every continent! � Fire, and the combustion of fossil fuels, is nothing but an extension of our metabolic processes by other means.
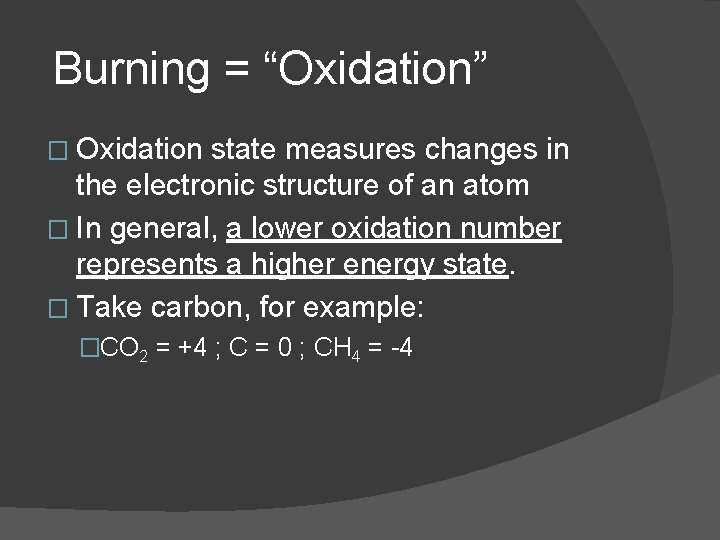
Burning = “Oxidation” � Oxidation state measures changes in the electronic structure of an atom � In general, a lower oxidation number represents a higher energy state. � Take carbon, for example: �CO 2 = +4 ; C = 0 ; CH 4 = -4
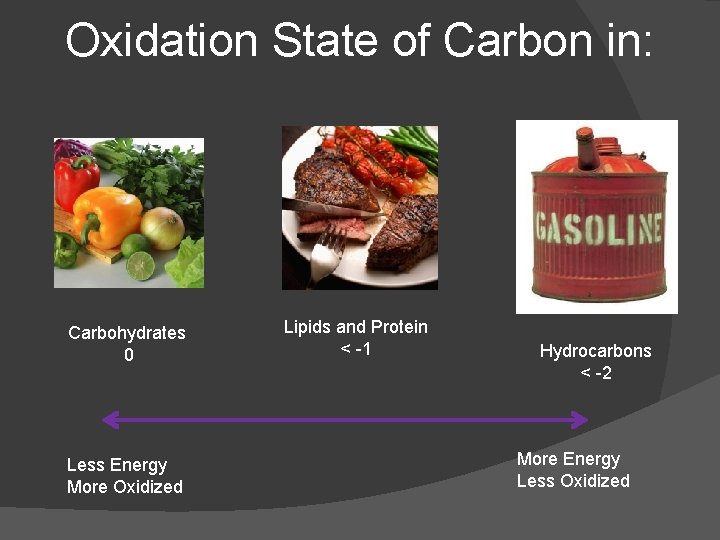
Oxidation State of Carbon in: Carbohydrates 0 Less Energy More Oxidized Lipids and Protein < -1 Hydrocarbons < -2 More Energy Less Oxidized

Oxidation and Reduction � Whenever something gets oxidized, the thing that oxidizes it gets reduced. � 2 Fe +3 O 2 2 Fe 2 O 3 � Before the reaction, Fe = 0, O = 0 � After, Fe = +3, O = -2 � For every yin, there is a yang.
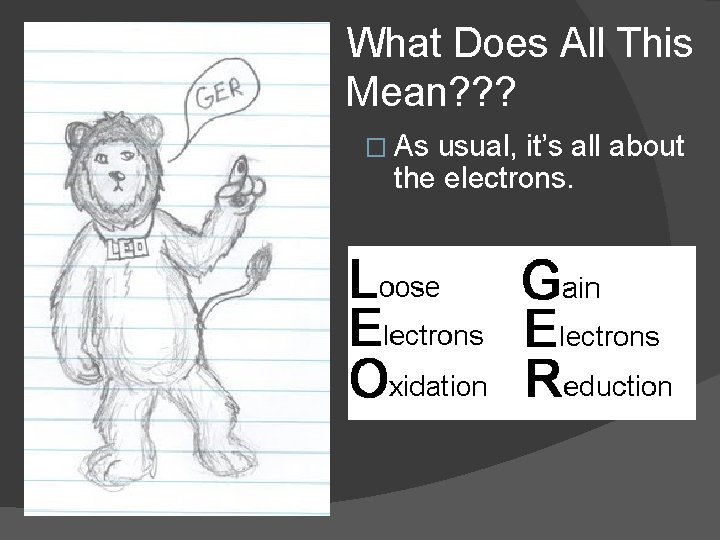
What Does All This Mean? ? ? � As usual, it’s all about the electrons.
So… The bottom line � When a substance is oxidized, it: �Releases energy (exothermic) �Looses electrons �Increases oxidation state � Carbon is oxidized when burned, or used in cellular respiration. � Carbon is reduced during photosynthesis.

Example: Burn Sugar and Follow the Carbon � C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O � Before the exothermic reaction, the carbon in C 6 H 12 O 6 has an oxidation number of 0. � During the reaction, carbon looses electrons. � After the reaction, the carbon (now in CO 2) has an oxidation number of +4. � Carbon has been oxidized.

Carbon Oxidation = Aerobic Life � The oxidation of carbon is the biochemical basis upon which almost all life rests. � The rate at which an individual or population can survive, grow, and reproduce is limited by how quickly it can get energy by oxidizing carbon.

The Fire Outside… � Fire greatly increased homo’s access to energy. � With it, humans and their ancestors could: �Cook food, making new sources of nutrition available �Heat shelters, allowing them to live in colder climates �Carry a portable source of light.
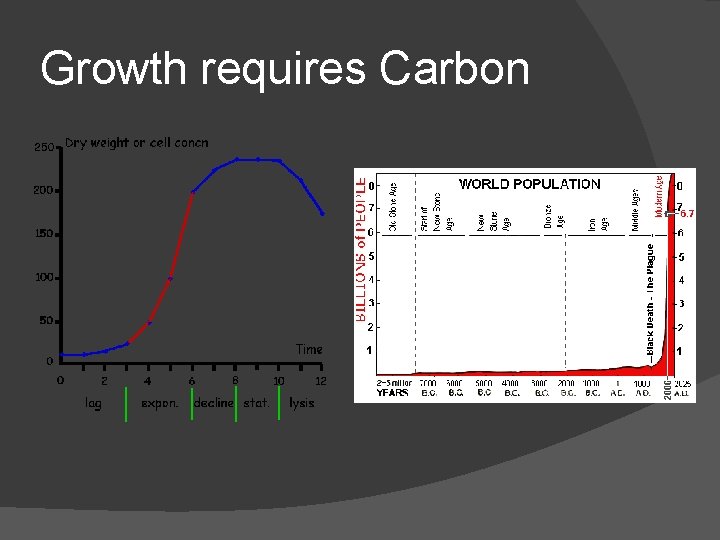
Growth requires Carbon
- Slides: 16