Do Now 1 st period Can you name


















- Slides: 30

Do Now – 1 st period Can you name the following? (Hint: Use prefixes on the second element) 1) CO Carbon monoxide 2) CO 2 Carbon dioxide 3) CCl 4 Carbon tetrachloride

Homework Achieve 3000 – Choose one of the following Earth From Above Another Earth "An hour for Earth" was a mistake! No activity, just an interesting read. . . Announcements Chapter 16 Test Next week Independent Work Packet 16. 2 In your notebook: Review Pages 360 (1 -8) and 368 (1 -8)

Do Now 1. What is an ion? 2. What is a polyatomic ion? 3. What is the net charge of a compound?

Homework Achieve 3000 – Choose one of the following “Earth From Above” or “Another Earth” Announcements Chapter 16 Test Either Fri/Mon Due Today Packet 16. 2 (late) In your notebook: Pages 360 (1 -8) and 368 (1 -8)

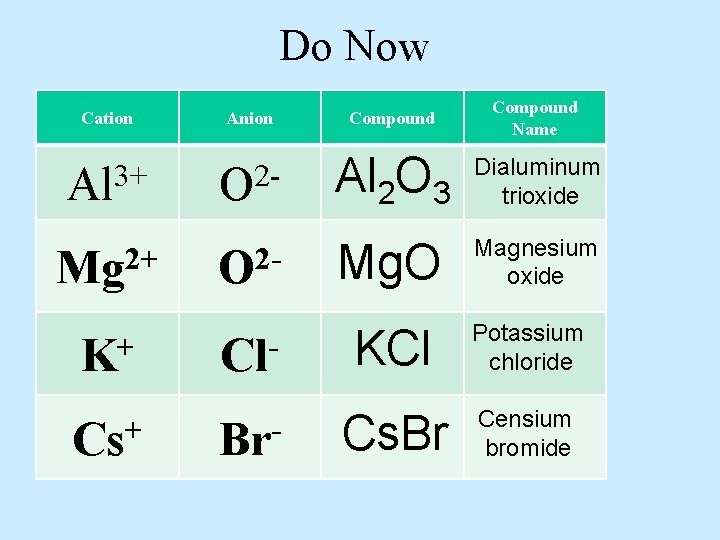
Do Now Cation Anion Compound Name 3+ Al 2 O 3 Dialuminum trioxide Mg. O Magnesium oxide Mg 2+ K+ Cs+ O 2 Cl. Br- KCl Potassium chloride Cs. Br Censium bromide

Do Now Can you name the following? (Hint: Use prefixes on the second element) 1) CO Carbon monoxide 2) CO 2 Carbon dioxide 3) CCl 4 Carbon tetrachloride

Homework Achieve 3000 – Choose one of the following “Earth From Above” or “Another Earth” "An hour for Earth" was a mistake! No activity, just an interesting read. . . Announcements Chapter 16 Test Next week Independent Work Packet 16. 2 Due Today Homework In your notebook: Pages 360 (1 -8) and 368 (1 -8)

Carbon Compounds L. O. : To determine the chemical formula of carbon compounds.

COUNTING ATOMS L. O. SWBAT identify the number of atoms for each element in a compound.

RULES FOR COUNTING ATOMS 1. SUBSCRIPTS only refer to the atom that they are to the right of. For example… H 2 S There are TWO atoms of HYDROGEN and only ONE atom of SULFUR.

LET’S PRACTICE! Mg. Cl 2 Atoms of Magnesium: 1 Atoms of Chlorine: 2 Al 2 S 3 Atoms of Aluminum: 2 Atoms of Sulfur: 3

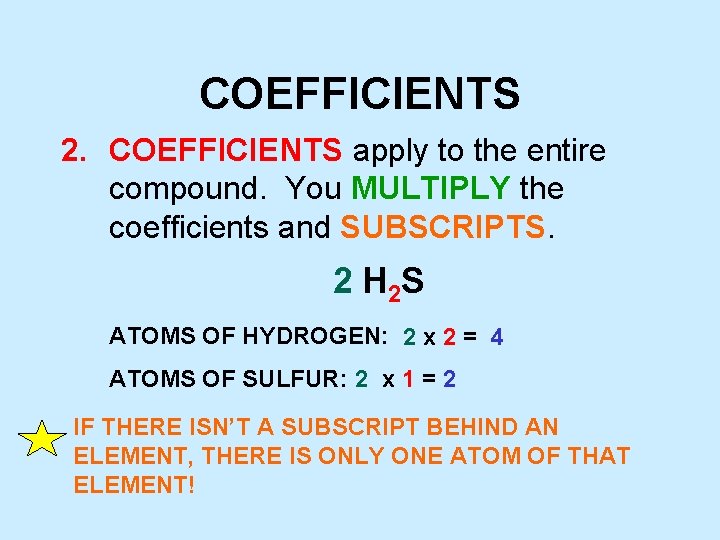
COEFFICIENTS 2. COEFFICIENTS apply to the entire compound. You MULTIPLY the coefficients and SUBSCRIPTS. 2 H 2 S ATOMS OF HYDROGEN: 2 x 2 = 4 ATOMS OF SULFUR: 2 x 1 = 2 IF THERE ISN’T A SUBSCRIPT BEHIND AN ELEMENT, THERE IS ONLY ONE ATOM OF THAT ELEMENT!

PRACTICE 2 H 2 SO 4 Atoms of Hydrogen: 4 Atoms of Sulfur: 2 Atoms of Oxygen: 8 3 CH 3 OH Atoms of Carbon: 3 Atoms of Hydrogen: 9 + 3 = 12 Atoms of Oxygen: 3

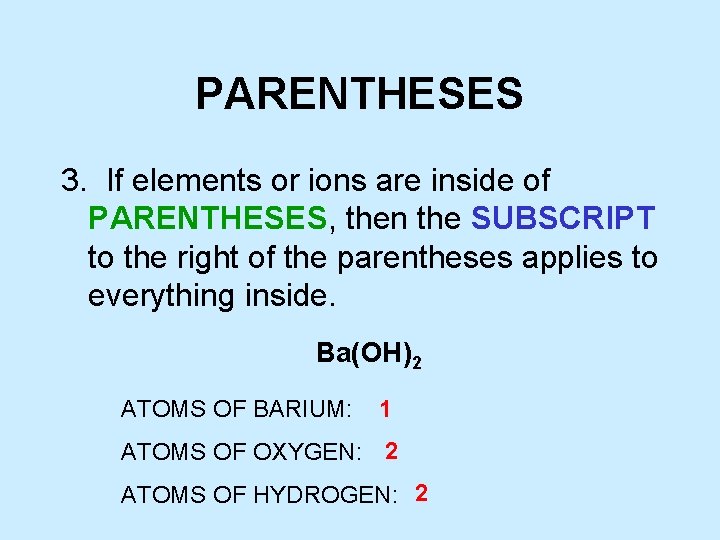
PARENTHESES 3. If elements or ions are inside of PARENTHESES, then the SUBSCRIPT to the right of the parentheses applies to everything inside. Ba(OH)2 ATOMS OF BARIUM: 1 ATOMS OF OXYGEN: 2 ATOMS OF HYDROGEN: 2

THIS COULD BE A LITTLE TRICKY… Ca 3(PO 4)2 Atoms of Calcium: 3 Atoms of Phosphorus: 2 Atoms of Oxygen: 8 Al 2(SO 4)3 Atoms of Aluminum: 2 Atoms of Sulfur: 3 Atoms of Oxygen: 12

What about this BAD BOY? ? ? 2 Ca 3(PO 4)2 Atoms of Calcium: 6 Atoms of Phosphorus: 4 Atoms of Oxygen: 16

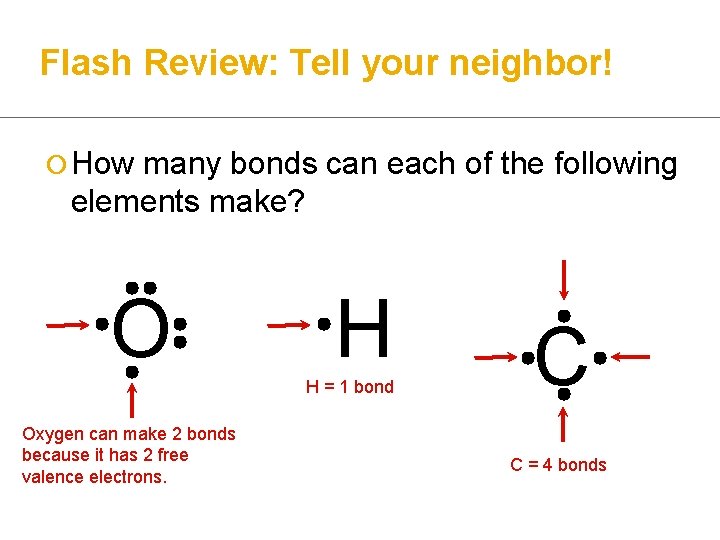
Flash Review: Tell your neighbor! ¡ How many bonds can each of the following elements make? O H H = 1 bond Oxygen can make 2 bonds because it has 2 free valence electrons. C C = 4 bonds

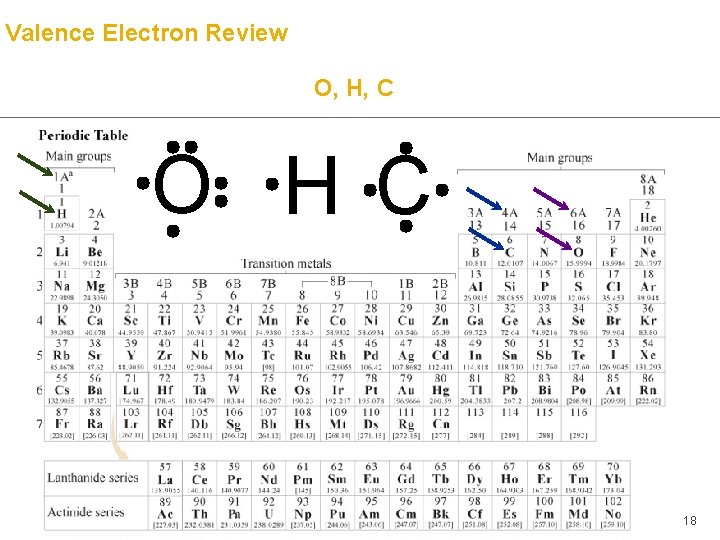
Valence Electron Review O, H, C O H C 18

Carbon ¡ can make 4 bonds with other elements in a compound. ¡ an essential element to all living things.

Carbon Bonds ¡ Carbon bonds: § Single bonds C C § Double bonds C C § Triple Bonds too! C C

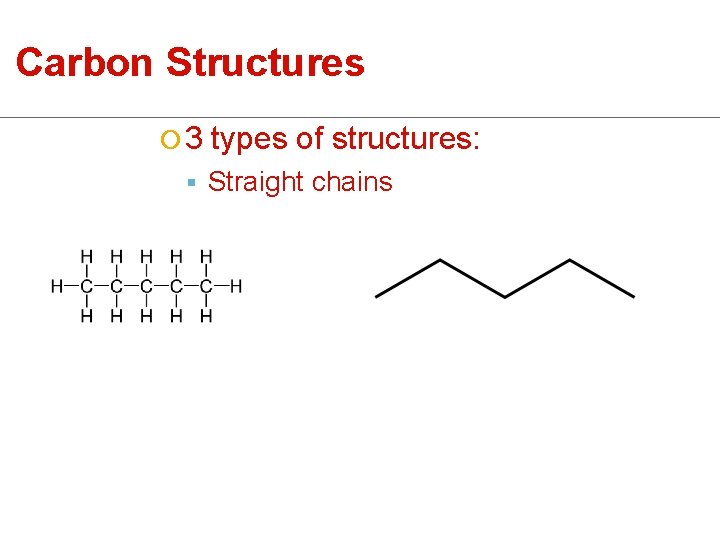
Carbon Structures ¡ 3 types of structures: § Straight chains

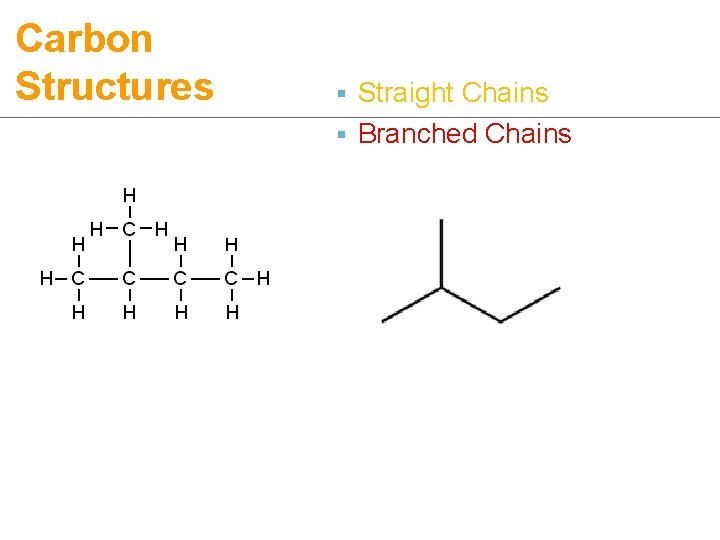
Carbon Structures § Straight Chains § Branched Chains

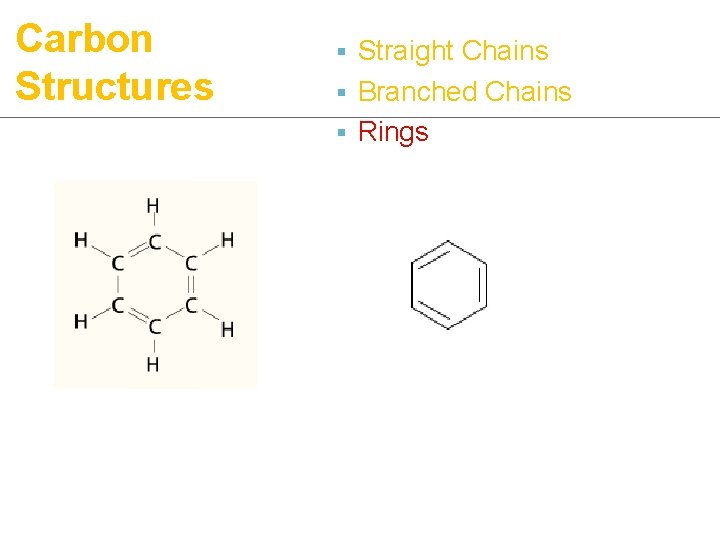
Carbon Structures § Straight Chains § Branched Chains § Rings

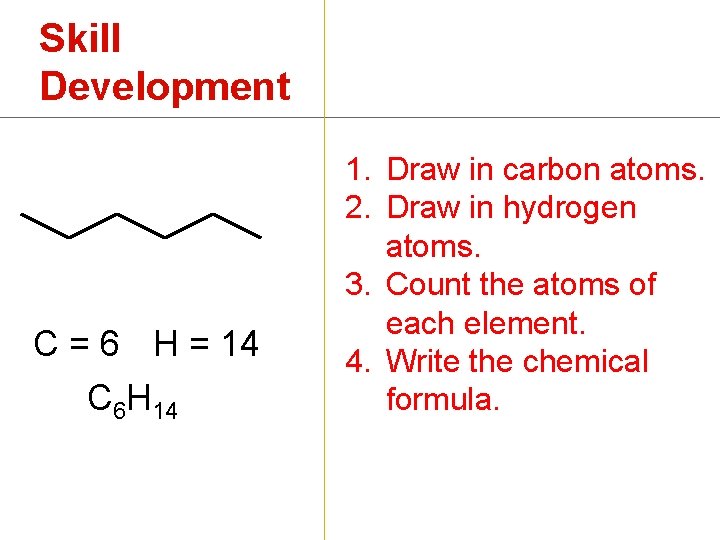
Skill Development C = 6 H = 14 C 6 H 14 1. Draw in carbon atoms. 2. Draw in hydrogen atoms. 3. Count the atoms of each element. 4. Write the chemical formula.

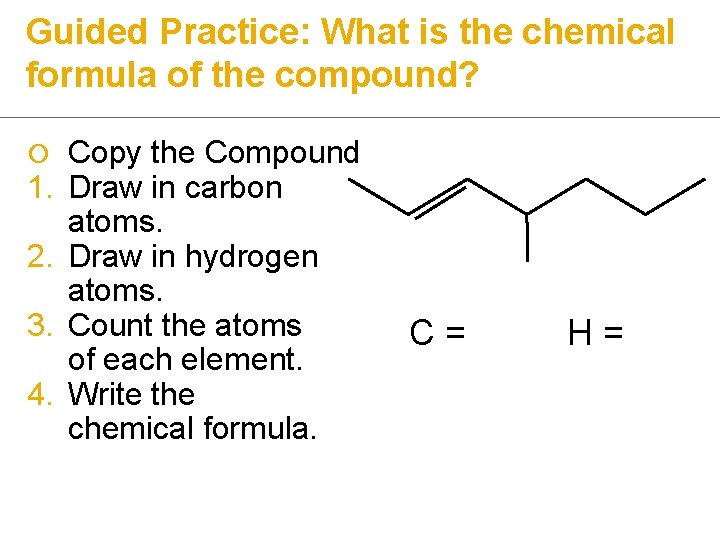
Guided Practice: What is the chemical formula of the compound? ¡ 1. 2. 3. 4. Copy the Compound Draw in carbon atoms. Draw in hydrogen atoms. Count the atoms of each element. Write the chemical formula. C= H=

Pair Share – Be ready to answer. ¡ Name three types of bonds carbon can use to bond to itself or other elements in a compound. Single bond Double bond Triple bond

Pair Share ¡ Why are carbon compounds so important to understand? Because carbon is an essential element to all living things.

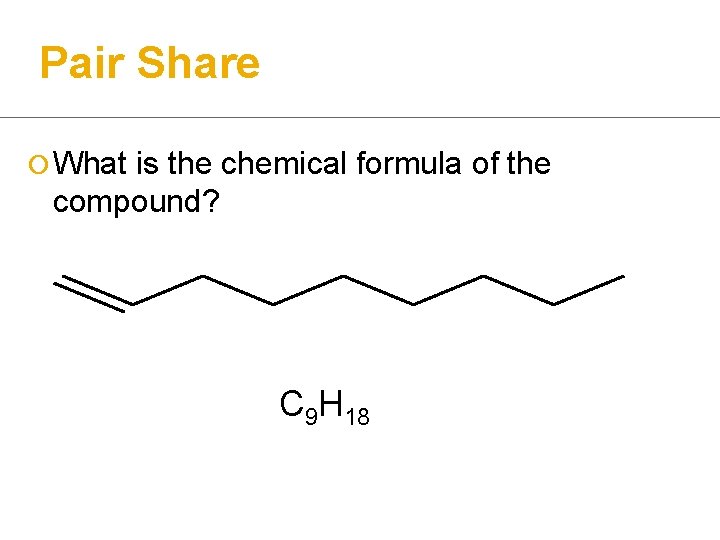
Pair Share ¡ What is the chemical formula of the compound? C 9 H 18

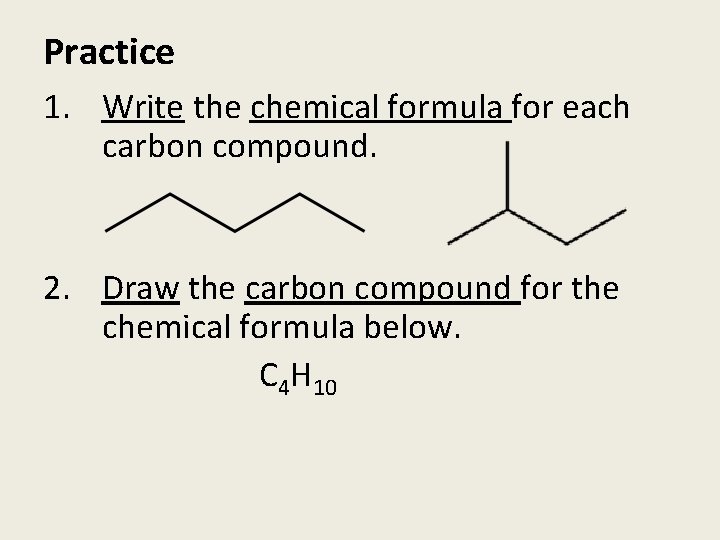
Practice 1. Write the chemical formula for each carbon compound. 2. Draw the carbon compound for the chemical formula below. C 4 H 10

Summary (2 -3 sentences) Why is carbon the most essential element for life?