Do Now 1 Label the element square 10

Do Now 1. Label the element square. 10 Ne Neon 20. 180 Atomic # Chemical Symbol Element Name Avg. Atomic mass # 2. What is an oxidation number? The charge of an atom when it gains, loses, or shares an e- in a chemical bond. 3. How do you think we can use these numbers to predict the type of bond formed? (ionic, covalent) Elements close to noble gasses tend to give or take e- and form ionic bonds.

Homework Achieve 3000 – A New House on the Block (4 -15) Announcements Friday – Take your child to work day Monday – FSA/FCAT Testing Week Packets and labs – make up work
L. O. SWBAT use the periodic table to predict compound names.

Glue in Oxidation Number Worksheet
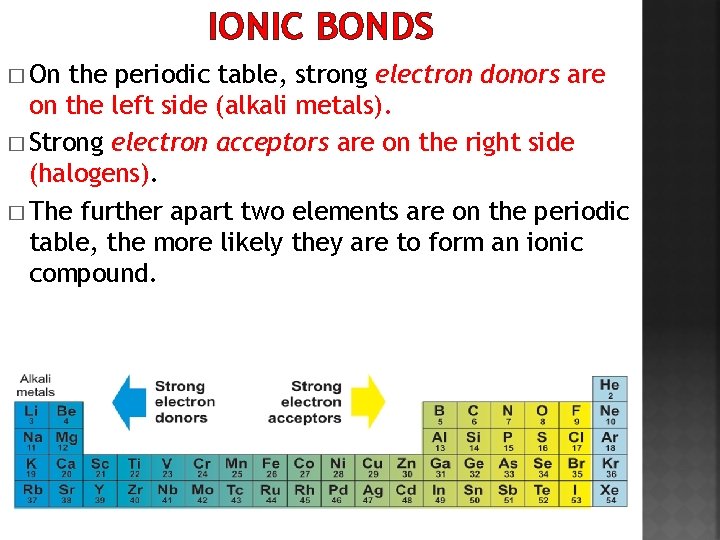
IONIC BONDS � On the periodic table, strong electron donors are on the left side (alkali metals). � Strong electron acceptors are on the right side (halogens). � The further apart two elements are on the periodic table, the more likely they are to form an ionic compound.
COVALENT BONDS � Form when electrons have equal tendency to accept e-. � Elements are both nonmetals and tend to be close together on the periodic table.
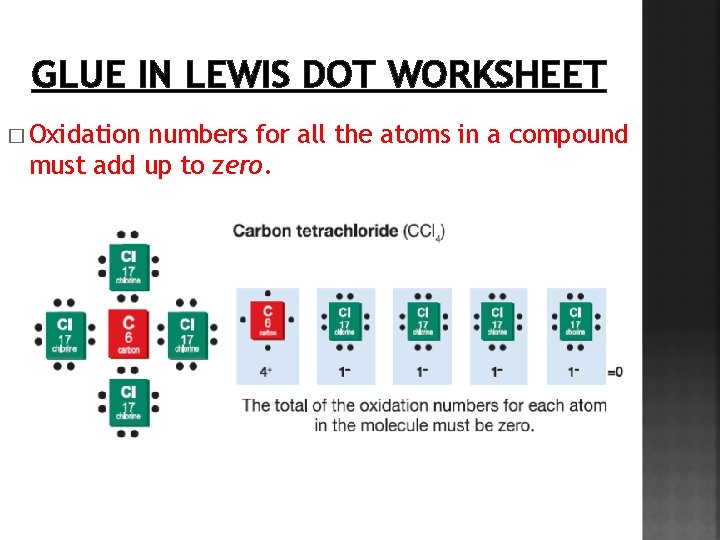
GLUE IN LEWIS DOT WORKSHEET � Oxidation numbers for all the atoms in a compound must add up to zero.
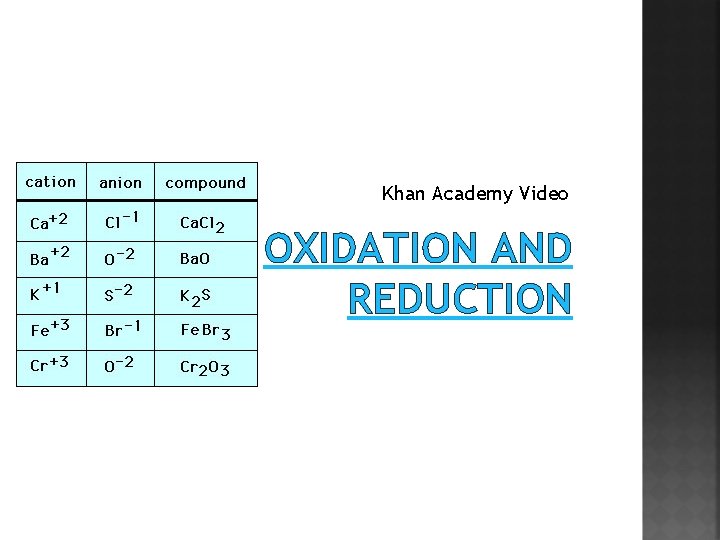
Khan Academy Video OXIDATION AND REDUCTION
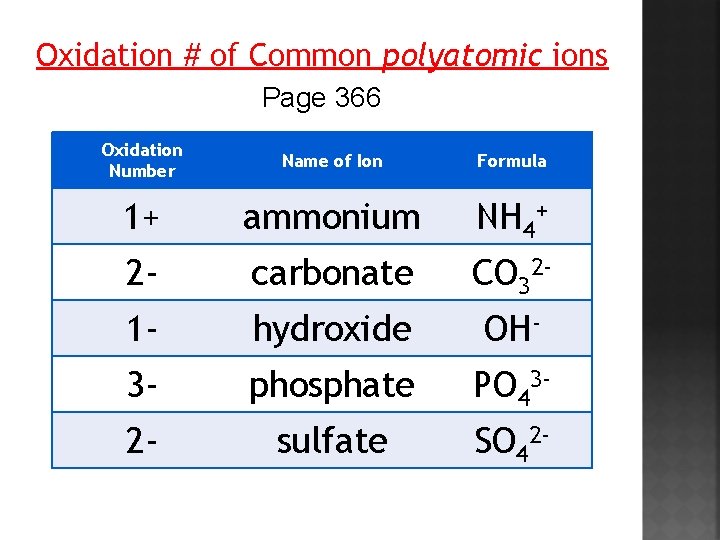
Oxidation # of Common polyatomic ions Page 366 Oxidation Number Name of Ion Formula 1+ ammonium NH 4+ 2 - carbonate CO 32 - 1 - hydroxide OH- 3 - phosphate PO 43 - 2 - sulfate SO 42 -

1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca
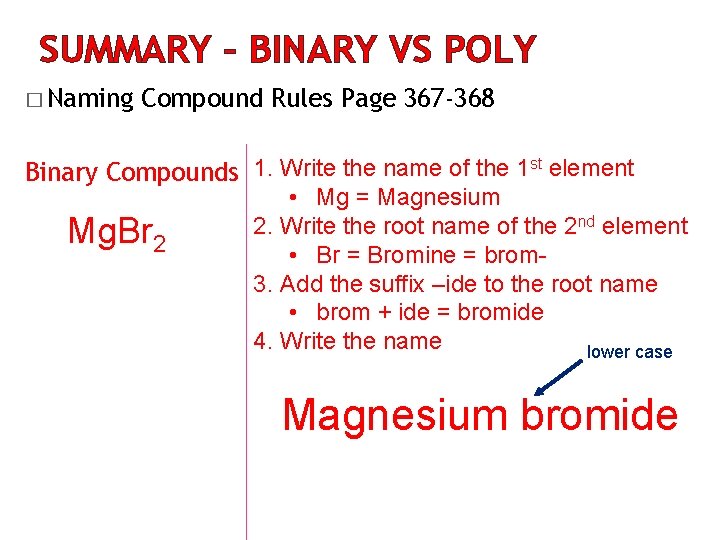
SUMMARY – BINARY VS POLY � Naming Compound Rules Page 367 -368 Binary Compounds 1. Write the name of the 1 st element Mg. Br 2 • Mg = Magnesium 2. Write the root name of the 2 nd element • Br = Bromine = brom 3. Add the suffix –ide to the root name • brom + ide = bromide 4. Write the name lower case Magnesium bromide
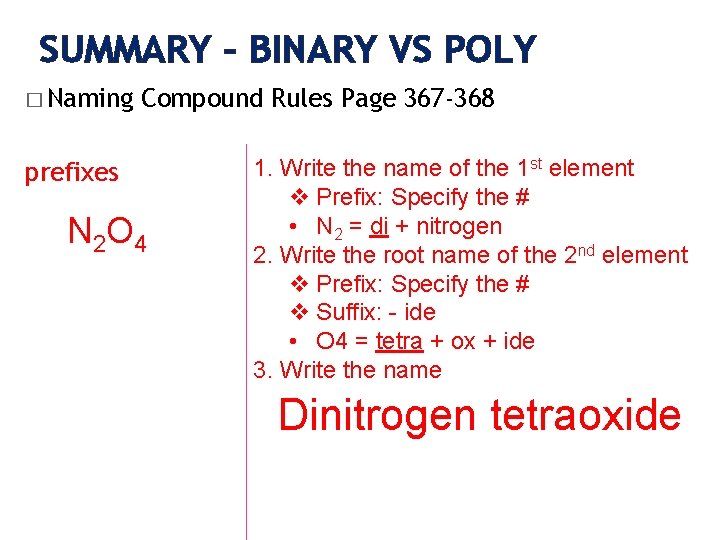
SUMMARY – BINARY VS POLY � Naming Compound Rules Page 367 -368 prefixes N 2 O 4 1. Write the name of the 1 st element v Prefix: Specify the # • N 2 = di + nitrogen 2. Write the root name of the 2 nd element v Prefix: Specify the # v Suffix: - ide • O 4 = tetra + ox + ide 3. Write the name Dinitrogen tetraoxide
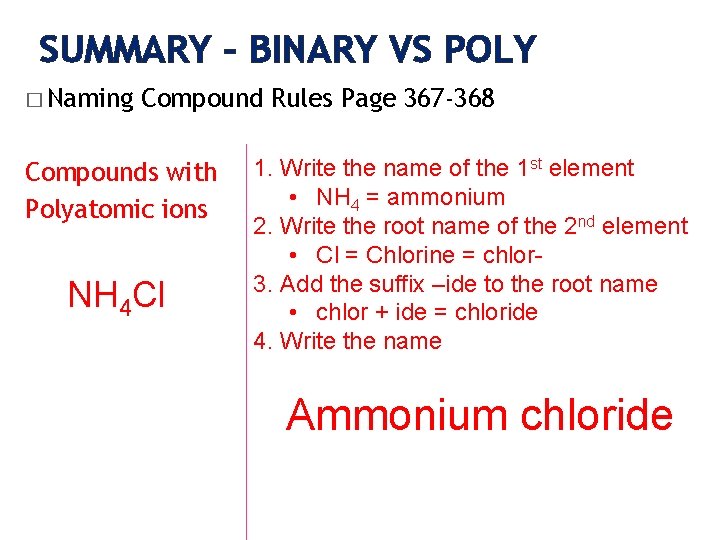
SUMMARY – BINARY VS POLY � Naming Compound Rules Page 367 -368 Compounds with Polyatomic ions NH 4 Cl 1. Write the name of the 1 st element • NH 4 = ammonium 2. Write the root name of the 2 nd element • Cl = Chlorine = chlor 3. Add the suffix –ide to the root name • chlor + ide = chloride 4. Write the name Ammonium chloride

Work on Packet 1 – Pre assessment and reading guide for chapter 16 section 1 Finished? Turn it in
- Slides: 15