DNVGL Medical Equipment and ISO 9001 Kelly Proctor















- Slides: 25

DNV-GL Medical Equipment and ISO 9001 Kelly Proctor CHFM, CHSP, CHOP DNV-GL Sector Lead Cell – 470 -553 -0670 1 DNV GL © 2014 SAFER, SMARTER, GREENER

About Kelly Proctor § Background in healthcare § CHFM, CHSP, CHOP § DNV-GL roles and responsibilities Surveyor Instructor Team Lead Physical Environment Sector Lead § Personal – Children, patents, Publications 2 DNV GL © 2014

About DNV-GL Accreditation § National Integrated Accreditation for Healthcare Organizations (NIAHO) § CMS Conditions of Participation (COP’s) § International Organization of Standardization (ISO) (NFPA, AAMI, ANSI, ASHRAE, ETC. ) ISO = A quality management program focused on risk based thinking and continual improvement (3 Years) 3 DNV GL © 2014

NIAHO® Physical Environment (PE) Management Systems ØPE. 1 Facility ØPE. 2 Life Safety Management System ØPE. 3 Safety Management System ØPE. 4 Security Management System ØPE. 5 Hazardous Material (Hazmat) Management System ØPE. 6 Emergency Management System ØPE. 7 Medical Equipment Management System ØPE. 8 Utility Management System DNV GL © 2014

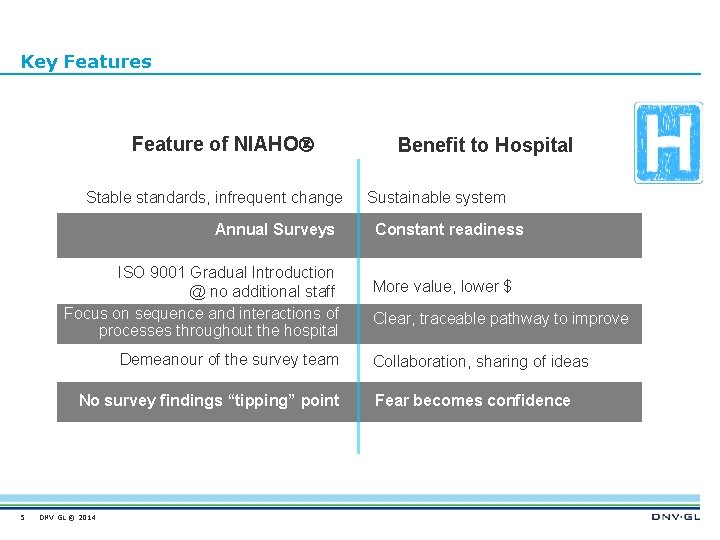
Key Features Feature of NIAHO Stable standards, infrequent change Annual Surveys ISO 9001 Gradual Introduction @ no additional staff Focus on sequence and interactions of processes throughout the hospital Demeanour of the survey team No survey findings “tipping” point 5 DNV GL © 2014 Benefit to Hospital Sustainable system Constant readiness More value, lower $ Clear, traceable pathway to improve Collaboration, sharing of ideas Fear becomes confidence

NIAHO® PE. 7: MEDICAL EQUIPMENT MANAGEMENT SYSTEM REQUIREMENTS SR. 1 The organization shall establish a Medical Equipment Management System that provides processes for the acquisition, safe use, and the appropriate selection of equipment. SR. 2 The Medical Equipment Management System shall address issues related to the organization’s initial service inspection, the orientation, and the demonstration of use for rental or physician owned equipment. SR. 3. The Medical Equipment Management System shall address criteria for the selection of equipment. SR. 4. The Medical Equipment Management System shall address incidents related to serious injury or illness or death (See SMDA 1990). DNV GL © 2014

NIAHO® PE. 7 MEDICAL EQUIPMENT MANAGEMENT SYSTEM SR. 5 The Medical Equipment Management System shall have a process for reporting and investigating equipment management problems, failures, and user errors. SR. 6 The Medical Equipment Management System shall address a process for determining timing and complexity of medical equipment maintenance. SR. 7 The Medical Equipment Management System shall address the process of receiving and responding to recalls and alerts. DNV GL © 2014

Alternative Equipment Maintenance Program ANSI/AAMI EQ 56: 1999/ (R) 2013, Recommended Practice for a Medical Equipment Management Program. § Decision to Place Equipment in an AEM Program § Maintenance requirements of the equipment § Equipment not Eligible for Placement in the AEM Program radiologic equipment medical laser device New equipment (new Technology) § Risk Assessment – History of failure for the equipment § Consequences of altering the frequency of the maintenance Make sure Leadership is aware 8 DNV GL © 2014

What is ISO 9001: 2015? ISO 9001: 2015 – Quality Management System with a focus on Risk Based Thinking and Continual Improvement. § Ensures the hospital considers risks § Ensures consistency § Ensures continual improvement § Ensures that data is analyzed § Ensures effective correction plans 9 DNV GL © 2014

About ISO 9001 Who Developed ISO 9001? – ISO 9001 was developed through the International Organization for Standardization – This organization began in 1946 and published the first revision of the ISO 9001 standard in 1987 – The current revision of ISO 9001 is the revision four dated 2015 – The 2015 9001 standard is a Quality Management System that requires the organization to perform risk based thinking 10 DNV GL © 2014

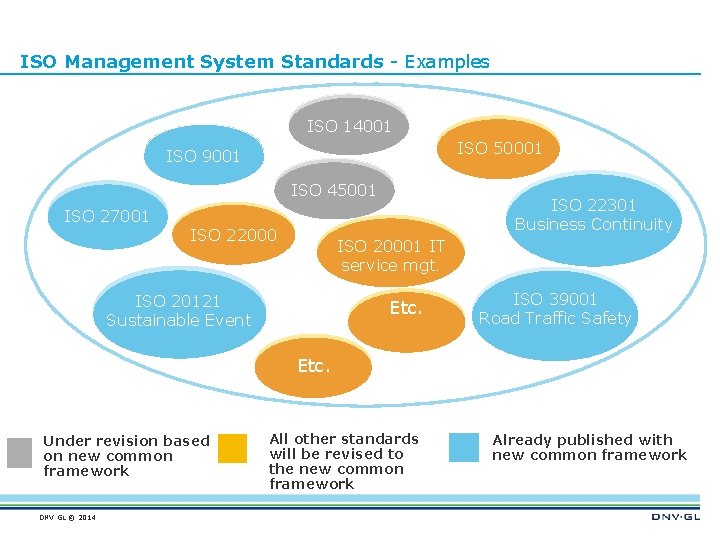
ISO Management System Standards - Examples ISO 14001 ISO 50001 ISO 9001 ISO 45001 ISO 27001 ISO 22000 ISO 22301 Business Continuity ISO 20001 IT service mgt. ISO 20121 Sustainable Event Etc. ISO 39001 Road Traffic Safety Etc. Under revision based on new common framework DNV GL © 2014 All other standards will be revised to the new common framework Already published with new common framework

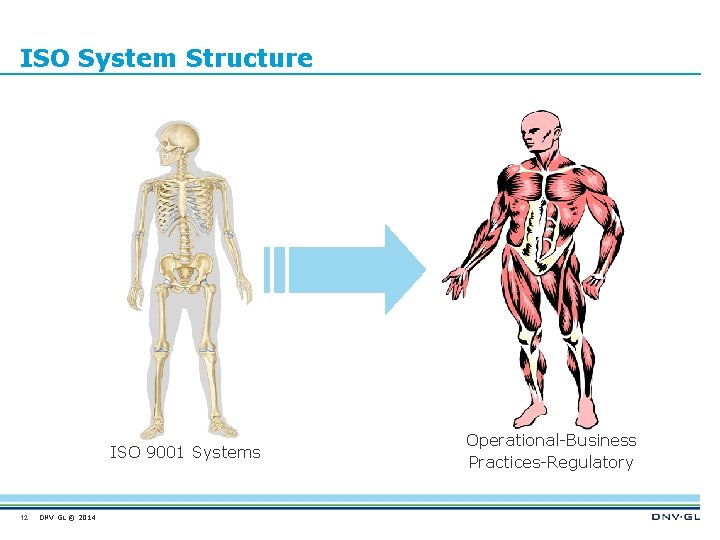
ISO System Structure ISO 9001 Systems 12 DNV GL © 2014 Operational-Business Practices-Regulatory

ISO 9001 Resource Management Concepts 7. 1. 6 Organizational knowledge 7. 2 Competence 7. 3 Awareness Personnel performing work affecting conformity to product requirements shall be competent on the basis of appropriate education, training, skills and experience. The organization determines the necessary competence for personnel performing work affecting conformity to product requirements, provides training or take other actions to achieve the necessary competence, evaluates the effectiveness of the actions taken, ensures that its personnel are aware of the relevance and importance of their activities and how they contribute to the achievement of the quality objectives, and maintains appropriate records of education, training, skills and experience. 13 DNV GL © 2014

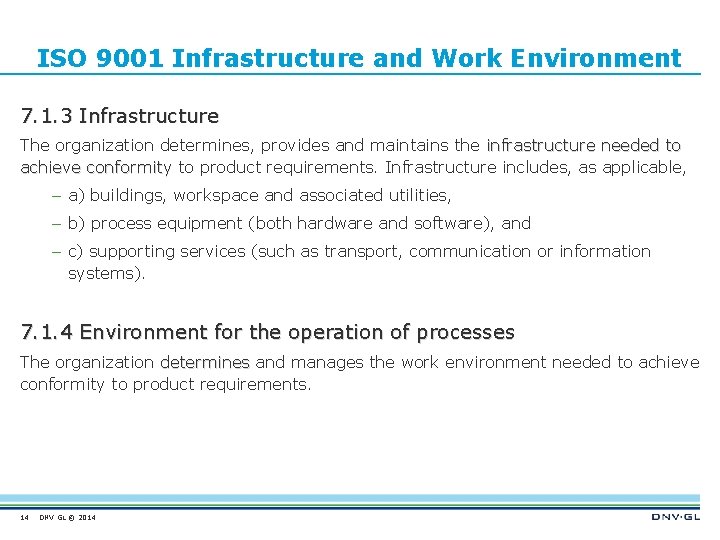
ISO 9001 Infrastructure and Work Environment 7. 1. 3 Infrastructure The organization determines, provides and maintains the infrastructure needed to achieve conformity to product requirements. Infrastructure includes, as applicable, – a) buildings, workspace and associated utilities, – b) process equipment (both hardware and software), and – c) supporting services (such as transport, communication or information systems). 7. 1. 4 Environment for the operation of processes The organization determines and manages the work environment needed to achieve conformity to product requirements. 14 DNV GL © 2014

ISO 9001 Control of Monitoring and Measuring Equipment is one of the major differences between DNV-GL and our competition. 15 DNV GL © 2014

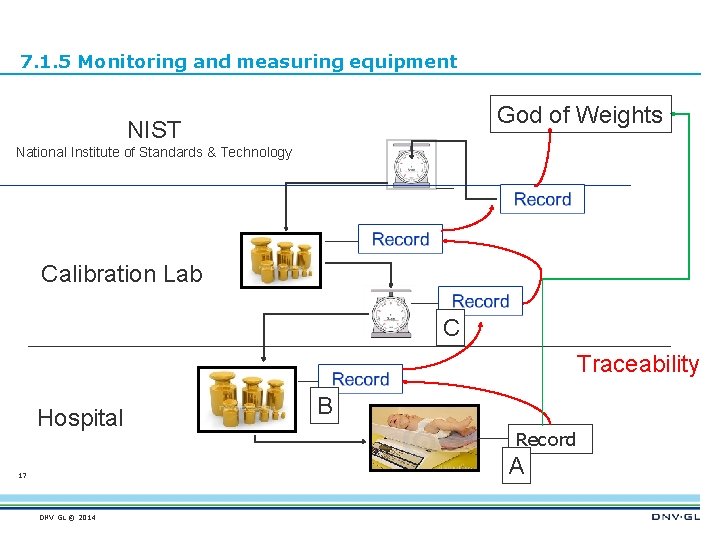
7. 1. 5 Monitoring and measuring resources The organization shall determine and provide the resources needed to ensure valid and reliable results when monitoring or measuring is used to verify the conformity of products and services to requirements. The organization shall ensure that the resources provided: a) are suitable for the specific type of monitoring and measurement activities being undertaken; b) are maintained to ensure their continuing fitness for their purpose. The organization shall retain appropriate documented information as evidence of fitness for purpose of the monitoring and measurement resources. 16 DNV GL © 2014

7. 1. 5 Monitoring and measuring equipment God of Weights NIST National Institute of Standards & Technology Calibration Lab C Traceability Hospital B Record A 17 DNV GL © 2014

ISO 9001: 2015 Communication 7. 4 Communication The organization ensures that responsibilities and authorities are defined and communicated within the organization. The organization ensures that appropriate communication processes are established within the organization and that communication takes place regarding the effectiveness of the quality management system 18 DNV GL © 2014

ISO 9001: 2015 Control 8. 4 Control of externally provided products and services 8. 4. 1 General The organization shall ensure that externally provided processes, products and services conform to requirements. The organization shall determine the controls to be applied to externally provided processes, products and services 8. 4. 2 Type and extent of control of external provision The organization shall ensure that externally provided processes, products and services do not adversely affect the organization’s ability to consistently deliver conforming products and services to its customers. 19 DNV GL © 2014

ISO 9001: 2015 Information 8. 4. 3 Information for external providers The organization ensures that purchased product conforms to specified purchase requirements. The type and extent of control applied to the supplier and the purchased product is dependent upon the effect of the purchased product on subsequent product realization or the final product. Purchasing information describes the product to be purchased – The organization establishes and implements the inspection or other activities necessary for ensuring that purchased product meets specified purchase requirements. – Where the organization or its customer intends to perform verification at the supplier's premises, premises the organization states the intended verification arrangements and method of product release in the purchasing information. 20 DNV GL © 2014

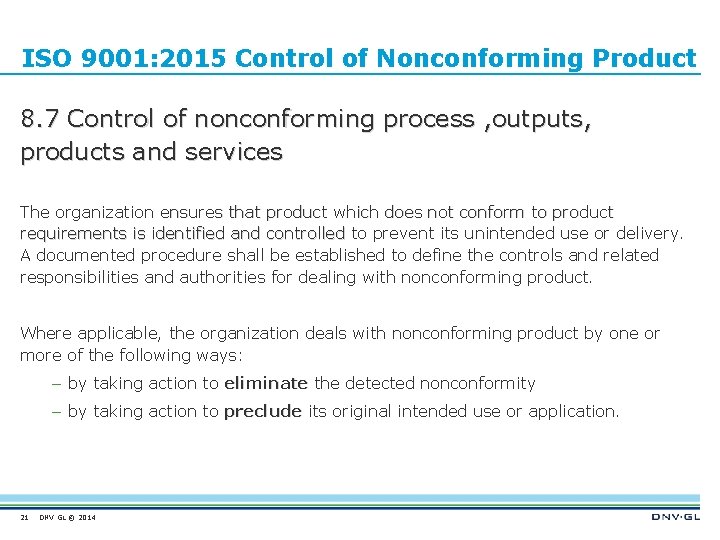
ISO 9001: 2015 Control of Nonconforming Product 8. 7 Control of nonconforming process , outputs, products and services The organization ensures that product which does not conform to product requirements is identified and controlled to prevent its unintended use or delivery. A documented procedure shall be established to define the controls and related responsibilities and authorities for dealing with nonconforming product. Where applicable, the organization deals with nonconforming product by one or more of the following ways: – by taking action to eliminate the detected nonconformity – by taking action to preclude its original intended use or application. 21 DNV GL © 2014

ISO 9001: 2015 9. 1. 3 Analysis and evaluation ISO 9001 9. 1. 3 Analysis and evaluation The organization shall analyze and evaluate appropriate data and information arising from monitoring and measurement. The results of analysis shall be used to evaluate: a) conformity of products and services; b) the degree of customer satisfaction; c) the performance and effectiveness of the quality management system; d) if planning has been implemented effectively; e) the effectiveness of actions taken to address risks and opportunities; f) the performance of external providers; g) the need for improvements to the quality management system. 22 DNV GL © 2014

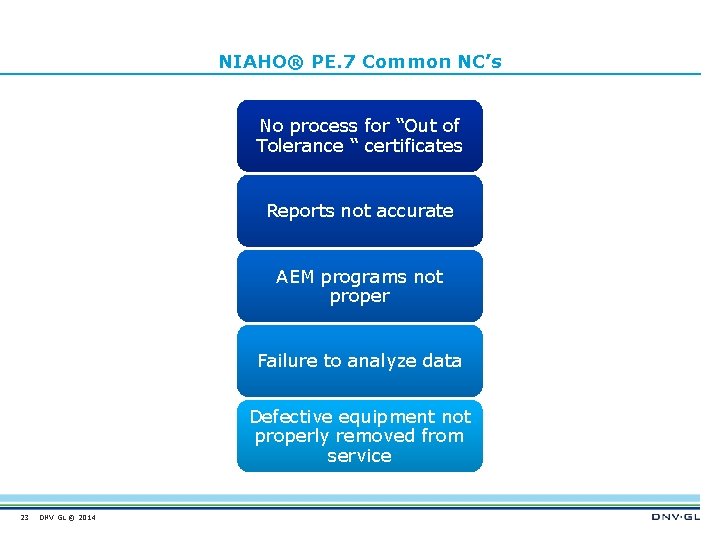
NIAHO® PE. 7 Common NC’s No process for “Out of Tolerance “ certificates Reports not accurate AEM programs not proper Failure to analyze data Defective equipment not properly removed from service 23 DNV GL © 2014

End of Day 2 DNV GL © 2014

Kelly Proctor CHFM, CHSP, CHOP Physical Environment Sector Lead Kelly. proctor@dnvgl. com www. dnvglhealthcare. com www. dnvgl. com SAFER, SMARTER, GREENER 25 DNV GL © 2014
 Norma iso 9000
Norma iso 9000 Ohsas 18001 iso 14001 comparison
Ohsas 18001 iso 14001 comparison Proctor medical equipment
Proctor medical equipment Iatf 16949 process interaction map
Iatf 16949 process interaction map Iso 9001 presentation
Iso 9001 presentation Iso 9001 history
Iso 9001 history May 2002 calendar
May 2002 calendar Agile iso 9001
Agile iso 9001 Software quality assurance iso standards
Software quality assurance iso standards Major changes in iso 9001 for 2015
Major changes in iso 9001 for 2015 Norma tecnica colombiana iso 9001
Norma tecnica colombiana iso 9001 Iso 9001:2000
Iso 9001:2000 Norme iso 9001 2008
Norme iso 9001 2008 Normas iso 9001:2008
Normas iso 9001:2008 Total quality management of kfc pdf
Total quality management of kfc pdf What is iso 20000 how does it relate to itil
What is iso 20000 how does it relate to itil Iso 9001:2015 management review meeting presentation ppt
Iso 9001:2015 management review meeting presentation ppt Ems iso 9001
Ems iso 9001 Iso 9001 introduction
Iso 9001 introduction Needs and expectations of interested parties example xls
Needs and expectations of interested parties example xls Iso 9001:2008 certification in mumbai
Iso 9001:2008 certification in mumbai Iso 9001 summary
Iso 9001 summary Iso 9001 2000 training
Iso 9001 2000 training Iso 37001 checklist
Iso 37001 checklist Iso 9001 drawing standards
Iso 9001 drawing standards Klausul 5 iso 9001
Klausul 5 iso 9001