DNA uptake during transformation of Bacillus subtilis Public

DNA uptake during transformation of Bacillus subtilis Public Health Research Institute and Department of Microbiology and Molecular Genetics University of Medicine and Dentistry Newark, New Jersey Look here throughout for the names of the people who did the work!

Not another transformation talk! Edvard Munch
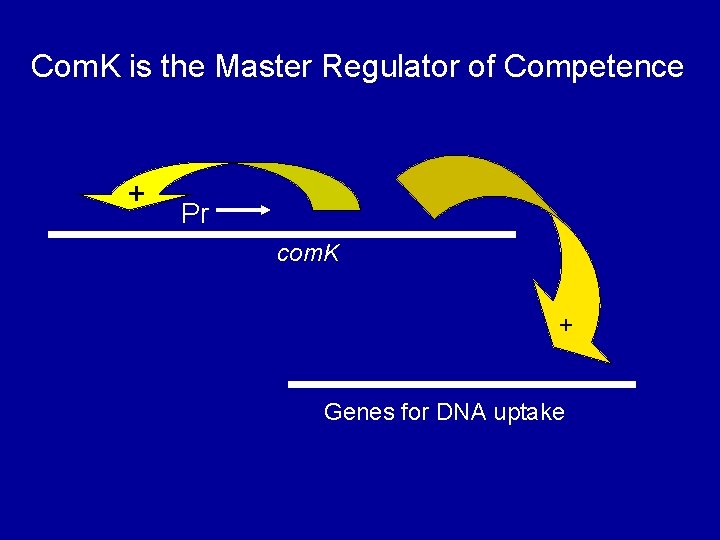
Com. K is the Master Regulator of Competence + Pr com. K + Genes for DNA uptake
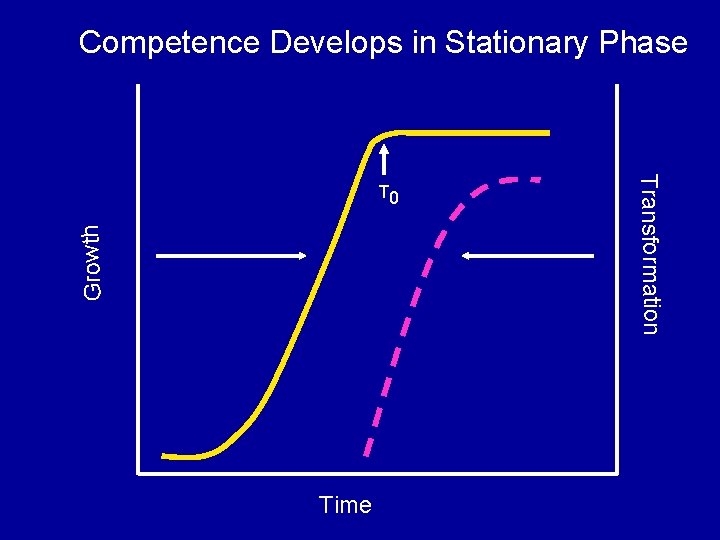
Competence Develops in Stationary Phase Growth Time Transformation T 0
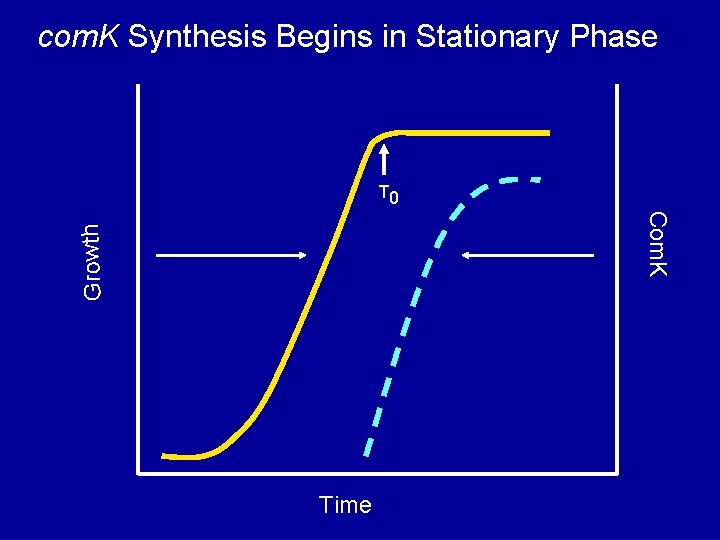
com. K Synthesis Begins in Stationary Phase T 0 Growth Com. K Time
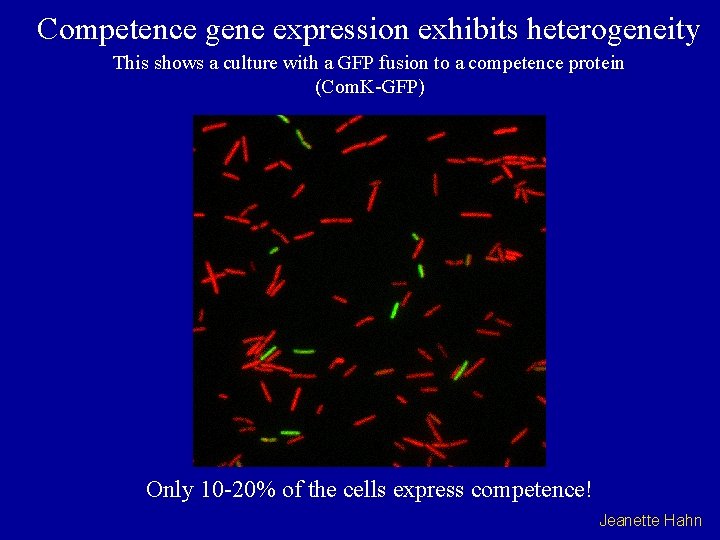
Competence gene expression exhibits heterogeneity This shows a culture with a GFP fusion to a competence protein (Com. K-GFP) Only 10 -20% of the cells express competence! Jeanette Hahn

DNA Uptake: take home lessons 1. DNA uptake is mediated by two subsets of proteins that probably form complexes. 2. The first subset provides for access across the wall. It involves a pilus-like system. 3. The second subset includes three proteins that mediate membrane transport. These proteins may constitute an atypical ABC transporter. 4. DNA uptake probably takes place preferentially at the cell poles and at least some competence proteins are localized at the poles. (All the proteins to be discussed are under Com. K control).
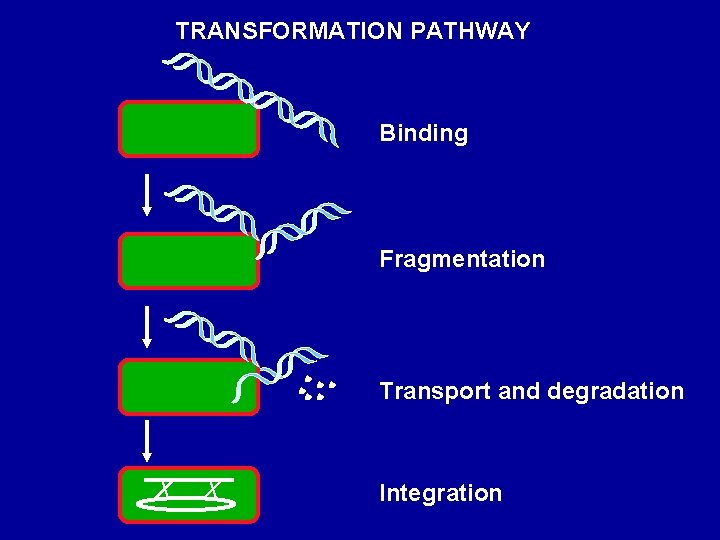
TRANSFORMATION PATHWAY Binding Fragmentation Transport and degradation Integration
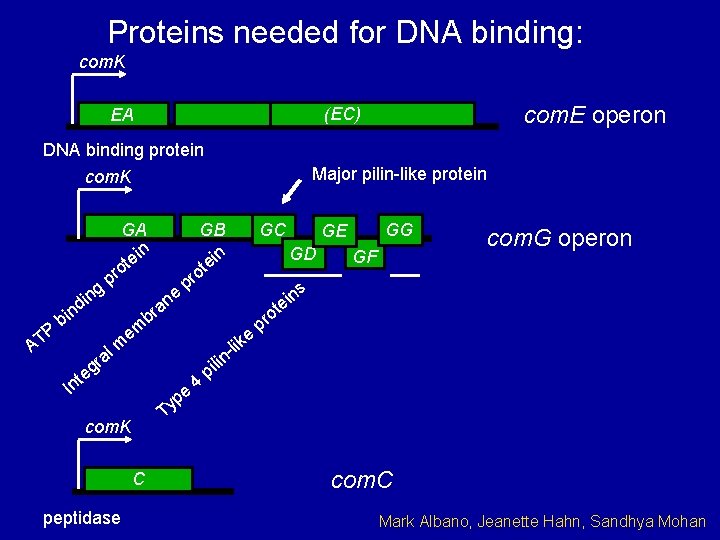
Proteins needed for DNA binding: com. K com. E operon (EC) EA DNA binding protein Major pilin-like protein com. K d P T A n bi g in r g e GA n ei t o pr ne GC p lm ns i e 4 lin i p e l- ik o pr Ty com. K C peptidase GF com. G operon t a pe GG GE GD a br em t In GB n ei t ro com. C Mark Albano, Jeanette Hahn, Sandhya Mohan
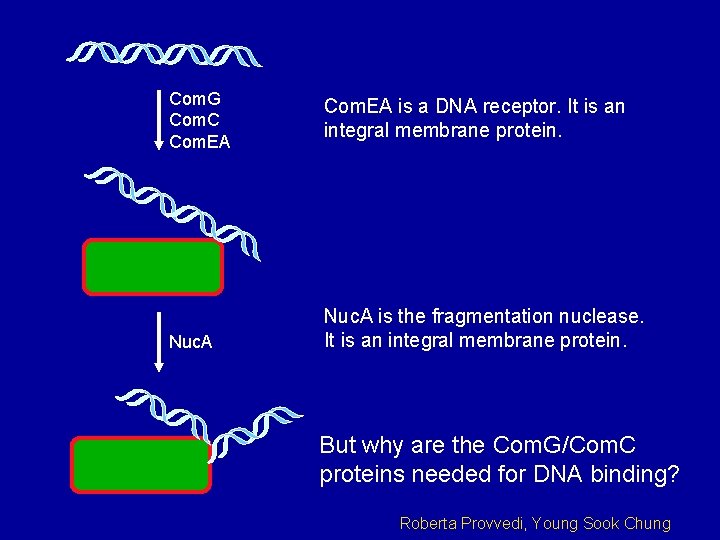
Com. G Com. C Com. EA Nuc. A Com. EA is a DNA receptor. It is an integral membrane protein. Nuc. A is the fragmentation nuclease. It is an integral membrane protein. But why are the Com. G/Com. C proteins needed for DNA binding? Roberta Provvedi, Young Sook Chung
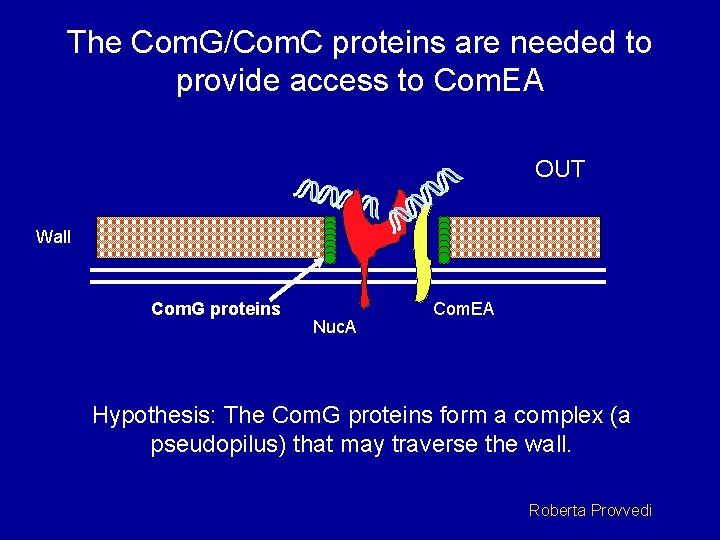
The Com. G/Com. C proteins are needed to provide access to Com. EA OUT Wall Com. G proteins Nuc. A Com. EA Hypothesis: The Com. G proteins form a complex (a pseudopilus) that may traverse the wall. Roberta Provvedi

Type IV pilins and pilin-like proteins hydrophobic core F prepilin peptidase cleavage Neisseria pili Klebsiella pseudopilus (Sauvonnet et al. EMBO J. (2000)19: 2221)
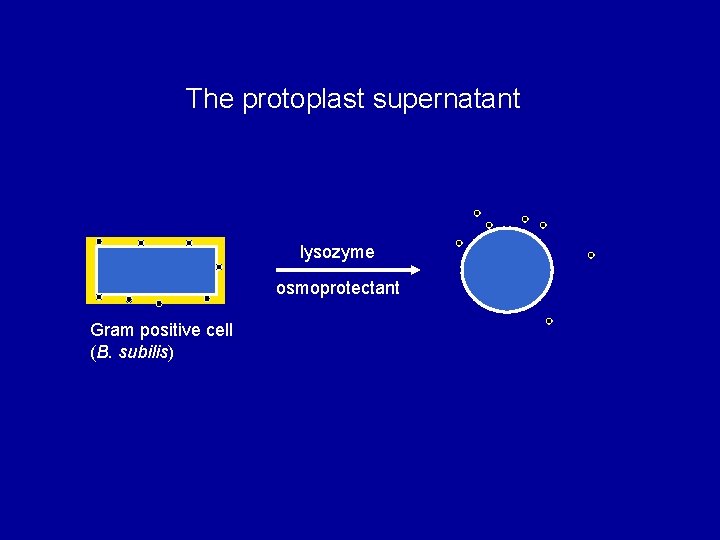
The protoplast supernatant lysozyme osmoprotectant Gram positive cell (B. subilis)
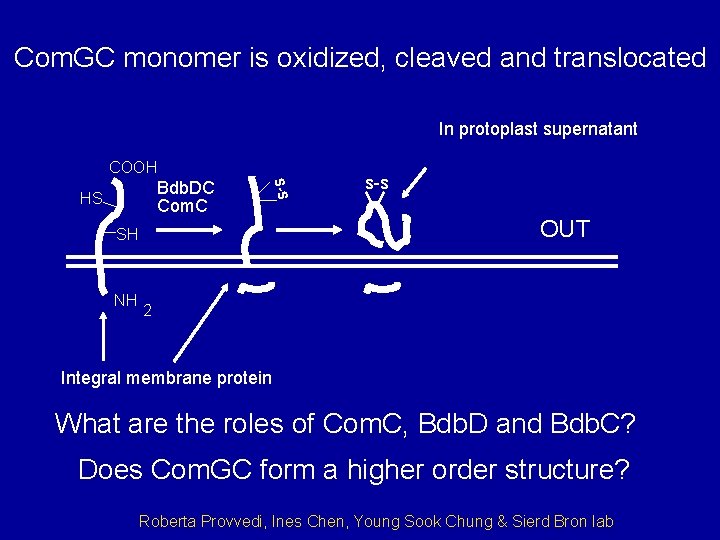
Com. GC monomer is oxidized, cleaved and translocated In protoplast supernatant COOH S-S Bdb. DC Com. C HS SH NH s-s OUT 2 Integral membrane protein What are the roles of Com. C, Bdb. D and Bdb. C? Does Com. GC form a higher order structure? Roberta Provvedi, Ines Chen, Young Sook Chung & Sierd Bron lab
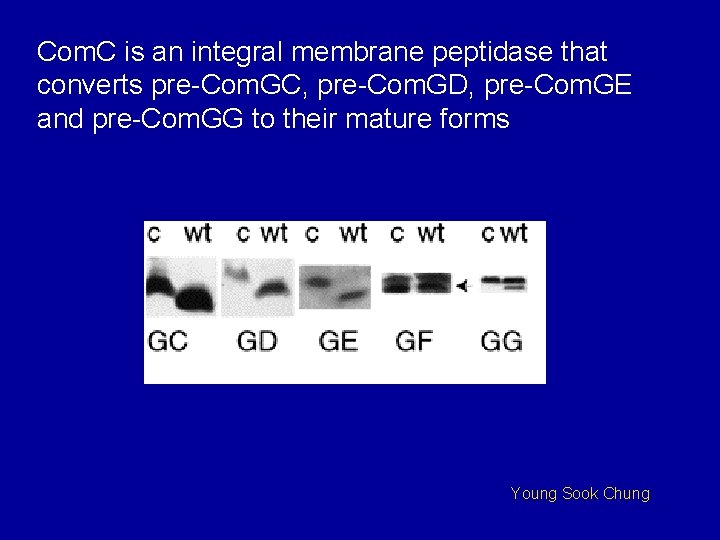
Com. C is an integral membrane peptidase that converts pre-Com. GC, pre-Com. GD, pre-Com. GE and pre-Com. GG to their mature forms Young Sook Chung
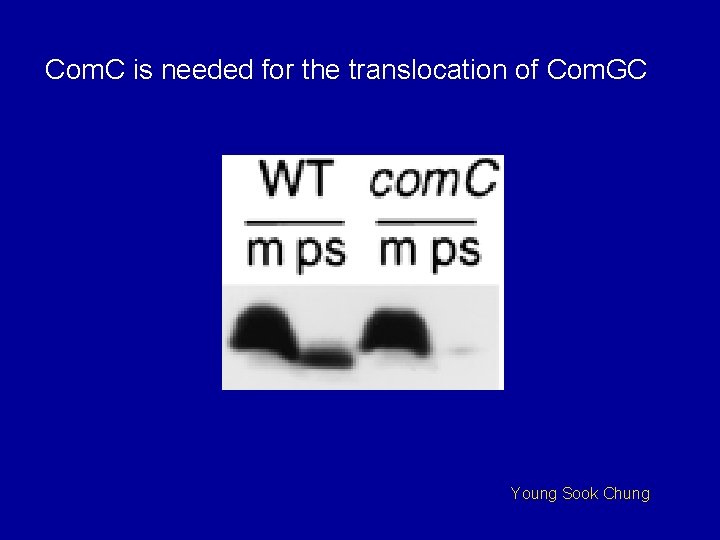
Com. C is needed for the translocation of Com. GC Young Sook Chung
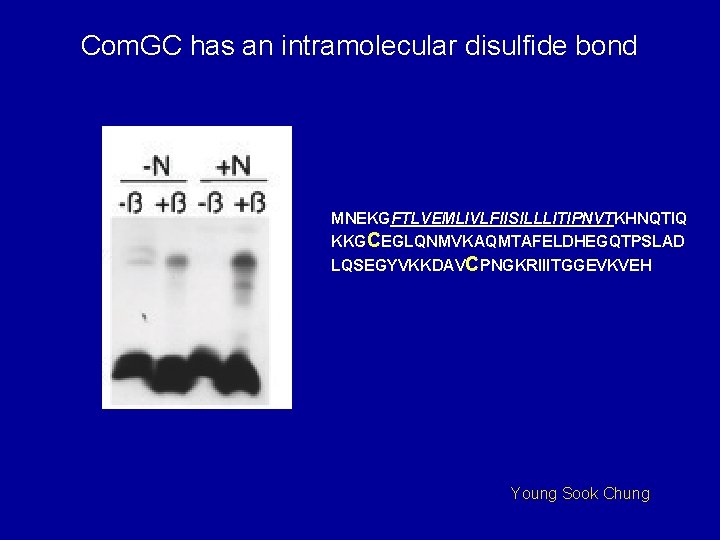
Com. GC has an intramolecular disulfide bond MNEKGFTLVEMLIVLFIISILLLITIPNVTKHNQTIQ KKGCEGLQNMVKAQMTAFELDHEGQTPSLAD LQSEGYVKKDAVCPNGKRIIITGGEVKVEH Young Sook Chung
Bdb. D and Bdb. C are thiol-disulfide oxidoreductases required for competence bdb. D bdb. C Com. K Sierd Bron lab, Mark Albano
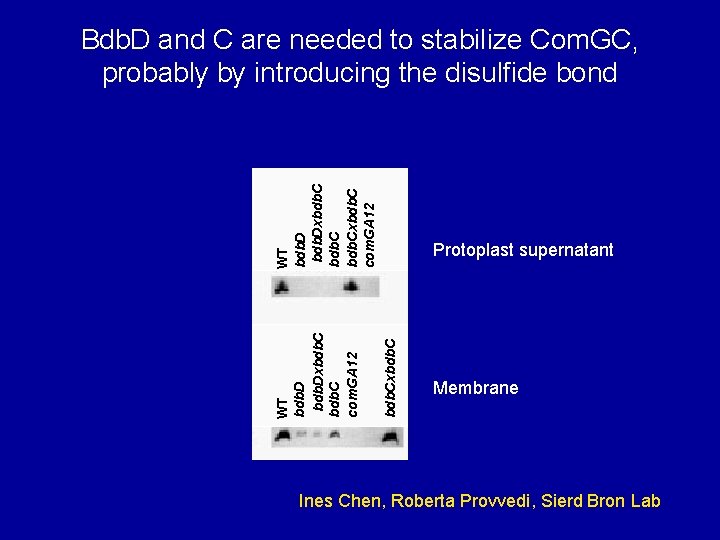
Protoplast supernatant bdb. Cxbdb. C WT bdb. Dxbdb. C com. GA 12 WT bdb. Dxbdb. Cxbdb. C com. GA 12 Bdb. D and C are needed to stabilize Com. GC, probably by introducing the disulfide bond Membrane Ines Chen, Roberta Provvedi, Sierd Bron Lab
Phenotypic suppression of bdb. D and bdb. C mutants Ines Chen
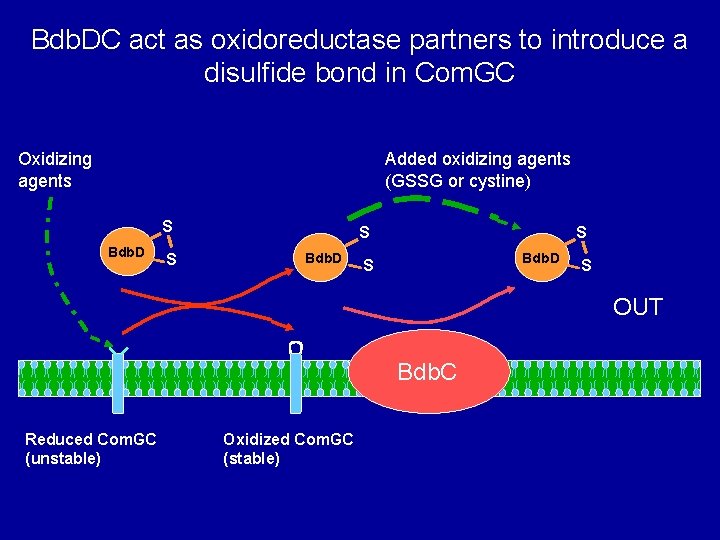
Bdb. DC act as oxidoreductase partners to introduce a disulfide bond in Com. GC Oxidizing agents Added oxidizing agents (GSSG or cystine) S Bdb. D S S OUT Bdb. C Reduced Com. GC (unstable) Oxidized Com. GC (stable)
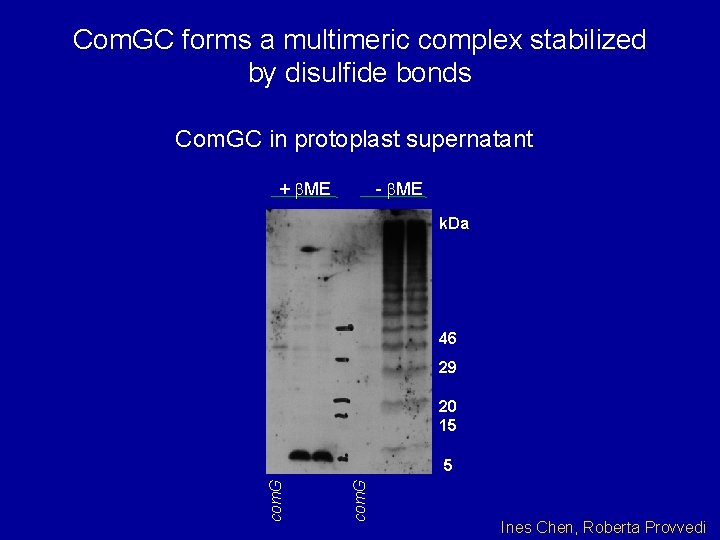
Com. GC forms a multimeric complex stabilized by disulfide bonds Com. GC in protoplast supernatant + b. ME - b. ME k. Da 46 29 20 15 com. G 5 Ines Chen, Roberta Provvedi
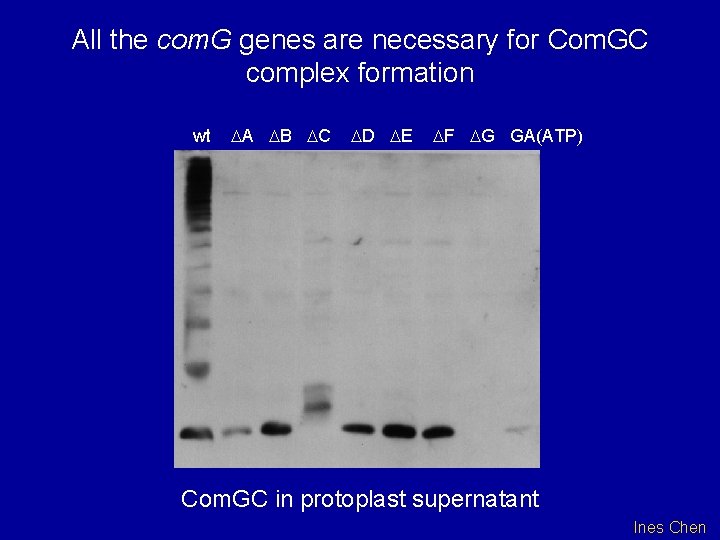
All the com. G genes are necessary for Com. GC complex formation wt DA DB DC DD DE DF DG GA(ATP) Com. GC in protoplast supernatant Ines Chen
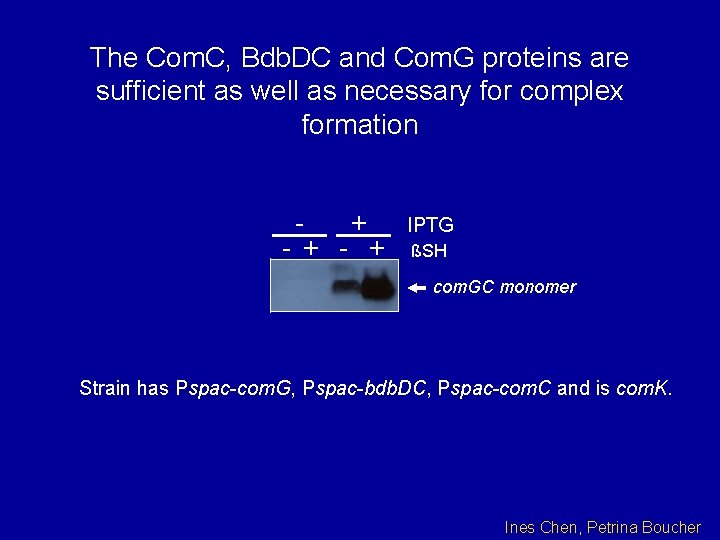
The Com. C, Bdb. DC and Com. G proteins are sufficient as well as necessary for complex formation - + + - + -+ - + IPTG ßSH com. GC monomer Strain has Pspac-com. G, Pspac-bdb. DC, Pspac-com. C and is com. K. Ines Chen, Petrina Boucher
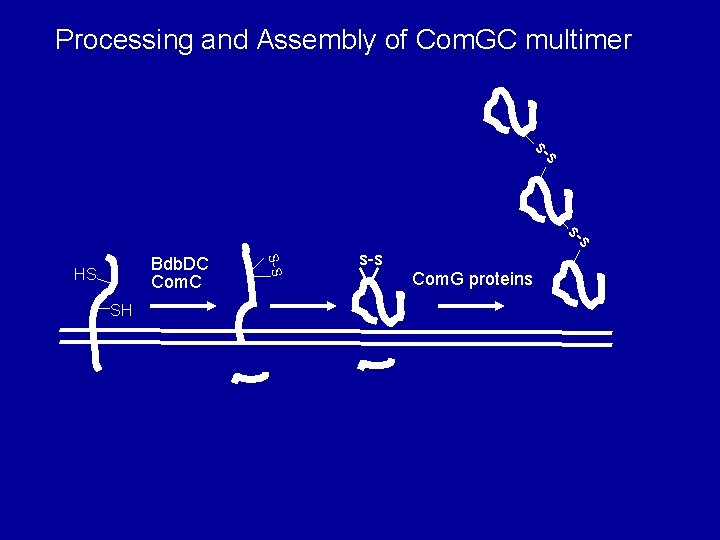
Processing and Assembly of Com. GC multimer s- s SH S-S Bdb. DC Com. C HS s-s Com. G proteins
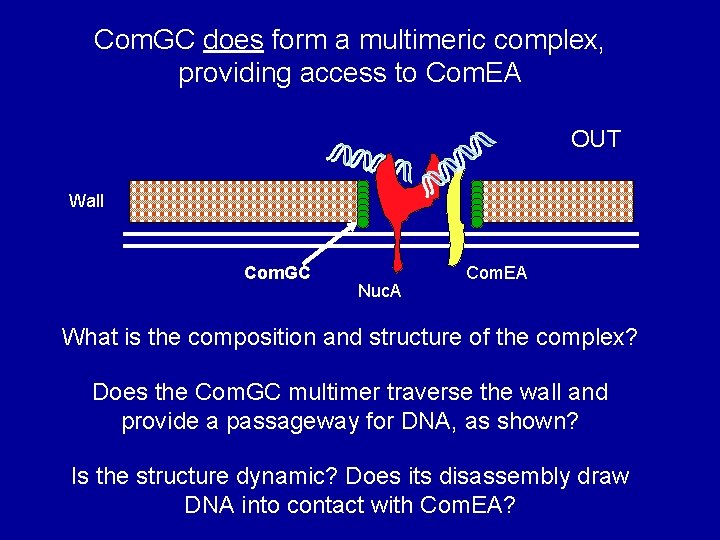
Com. GC does form a multimeric complex, providing access to Com. EA OUT Wall Com. GC Nuc. A Com. EA What is the composition and structure of the complex? Does the Com. GC multimer traverse the wall and provide a passageway for DNA, as shown? Is the structure dynamic? Does its disassembly draw DNA into contact with Com. EA?
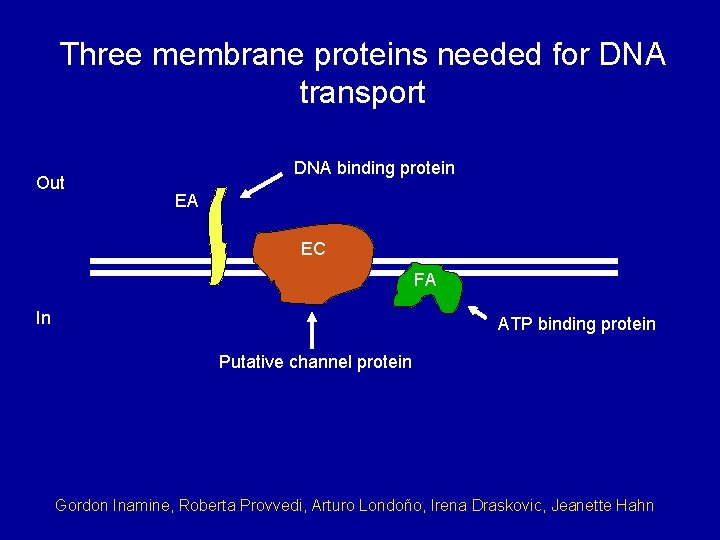
Three membrane proteins needed for DNA transport Out DNA binding protein EA EC FA In ATP binding protein Putative channel protein Gordon Inamine, Roberta Provvedi, Arturo Londoño, Irena Draskovic, Jeanette Hahn
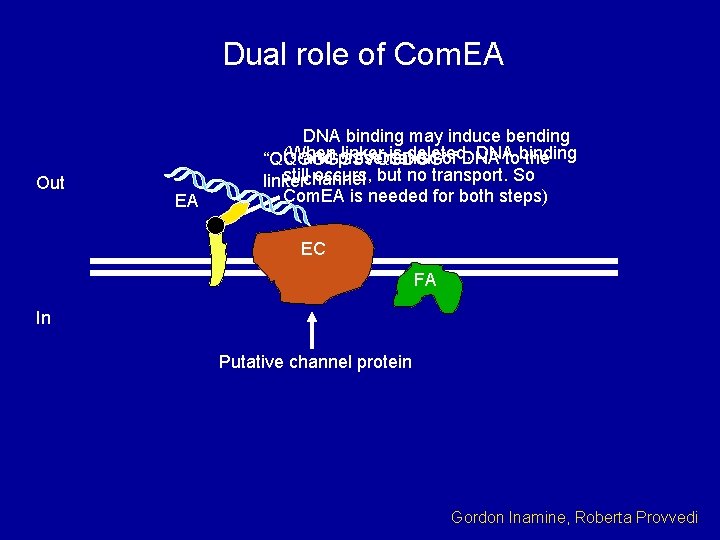
Dual role of Com. EA Out EA DNA binding may induce bending (When linker is deleted, DNAtobinding and presentation of DNA the “QQGGGGSVQSDGG” stillchannel occurs, but no transport. So linker Com. EA is needed for both steps) EC FA In Putative channel protein Gordon Inamine, Roberta Provvedi
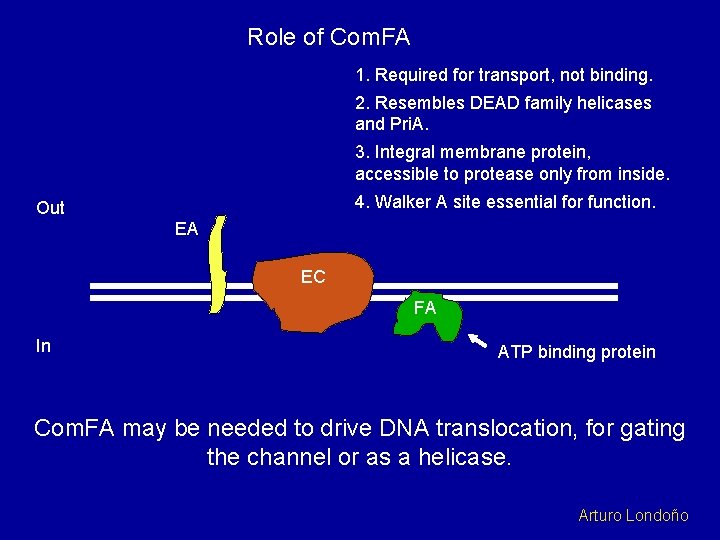
Role of Com. FA 1. Required for transport, not binding. 2. Resembles DEAD family helicases and Pri. A. 3. Integral membrane protein, accessible to protease only from inside. 4. Walker A site essential for function. Out EA EC FA In ATP binding protein Com. FA may be needed to drive DNA translocation, for gating the channel or as a helicase. Arturo Londoño
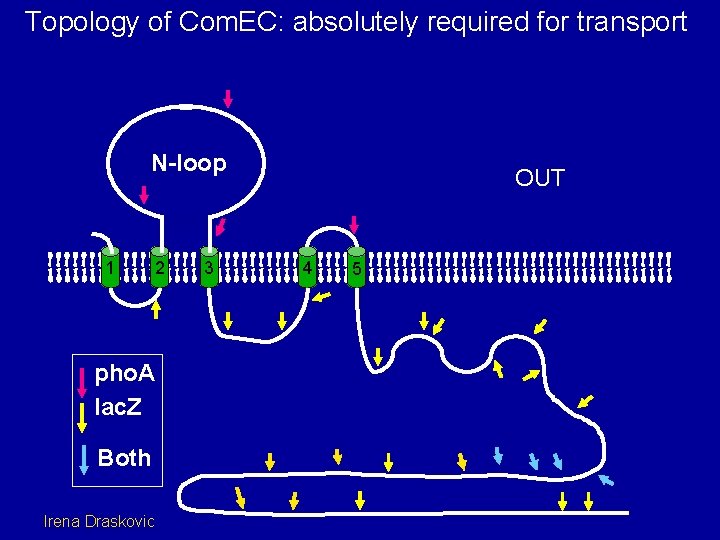
Topology of Com. EC: absolutely required for transport N-loop 1 2 pho. A lac. Z Both Irena Draskovic 3 OUT 4 5
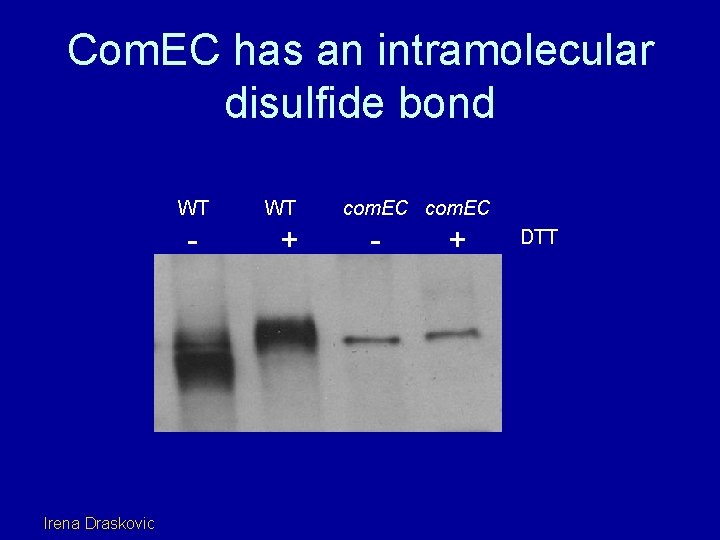
Com. EC has an intramolecular disulfide bond WT - Irena Draskovic WT + com. EC - + DTT
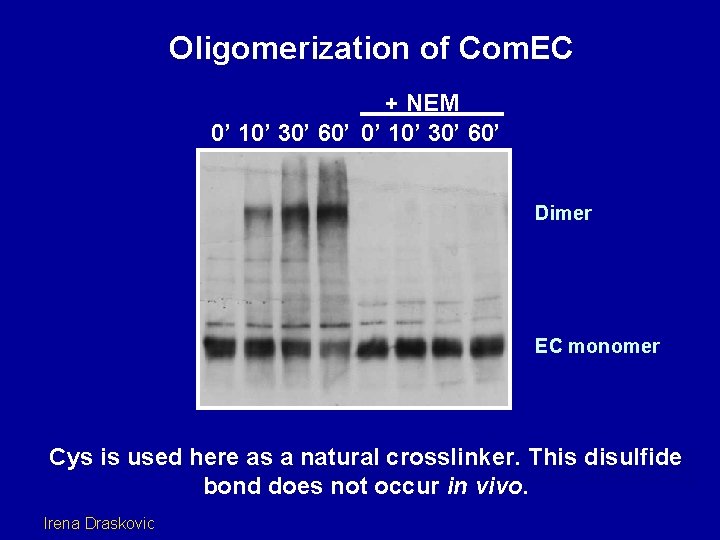
Oligomerization of Com. EC + NEM 0’ 10’ 30’ 60’ Dimer EC monomer Cys is used here as a natural crosslinker. This disulfide bond does not occur in vivo. Irena Draskovic

Which cysteine residues are responsible for the in vivo intramolecular disulfide bond and for the in vitro cross-linking? Irena Draskovic
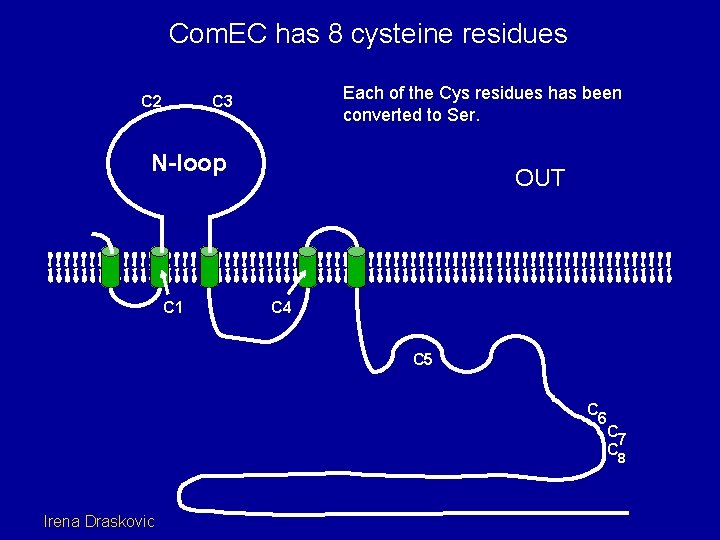
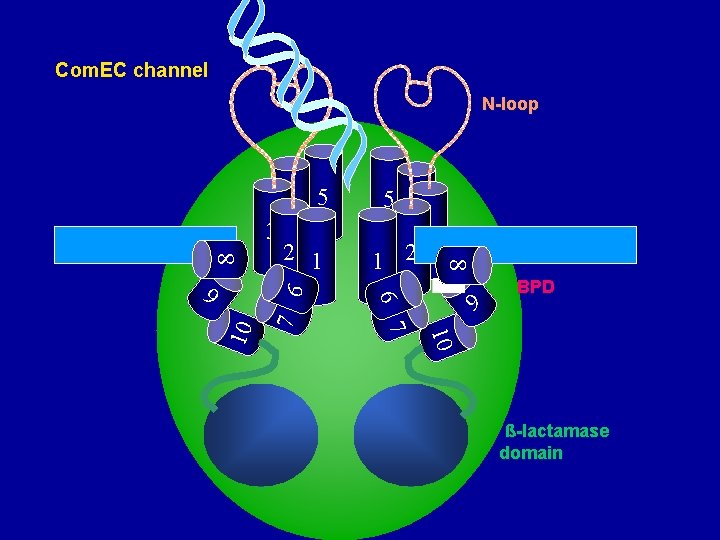
Com. EC has 8 cysteine residues C 2 Each of the Cys residues has been converted to Ser. C 3 N-loop C 1 OUT C 4 C 5 C 6 C 7 C 8 Irena Draskovic
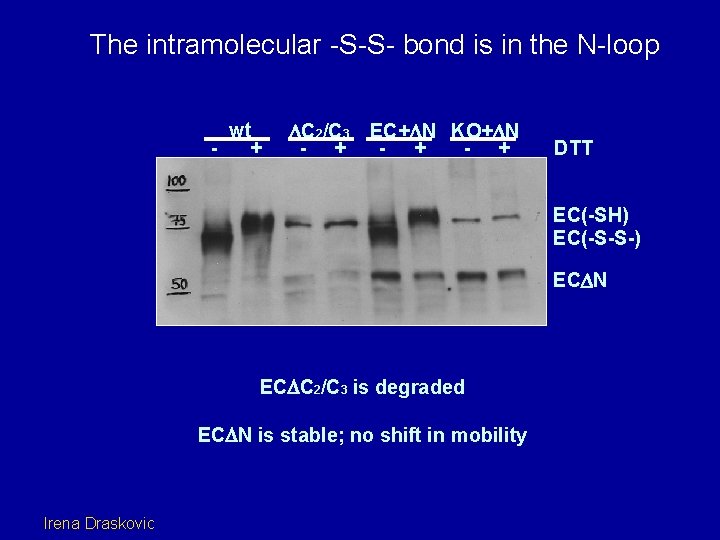
The intramolecular -S-S- bond is in the N-loop C 2 and C 3 form an internal disulfide bond wt + C 2/C 3 - + EC+ N KO+ N - + DTT EC(-SH) EC(-S-S-) EC N EC C 2/C 3 is degraded EC N is stable; no shift in mobility Irena Draskovic
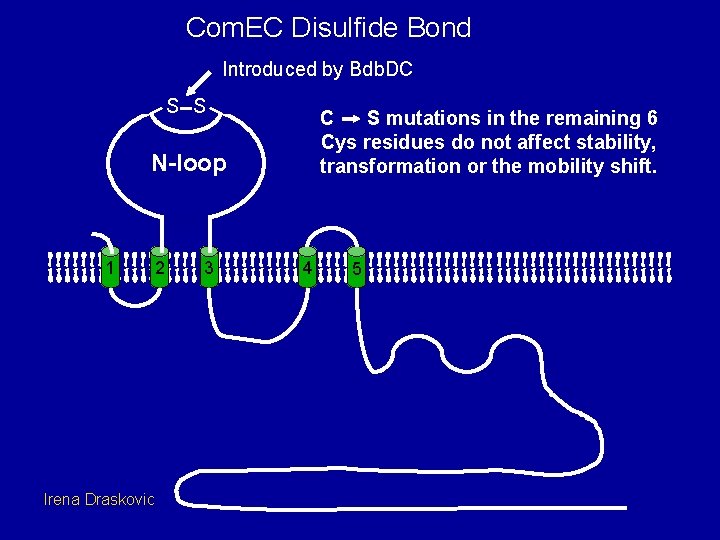
Com. EC Disulfide Bond Introduced by Bdb. DC S S C S mutations in the remaining 6 Cys residues do not affect stability, transformation or the mobility shift. N-loop 1 Irena Draskovic 2 3 4 5

Bdb. D and C are needed to introduce the N-loop disulfide bond wt DTT - + bdb. DC com. G com. C - + - + EC (-SH) EC (-S-S-) Irena Draskovic
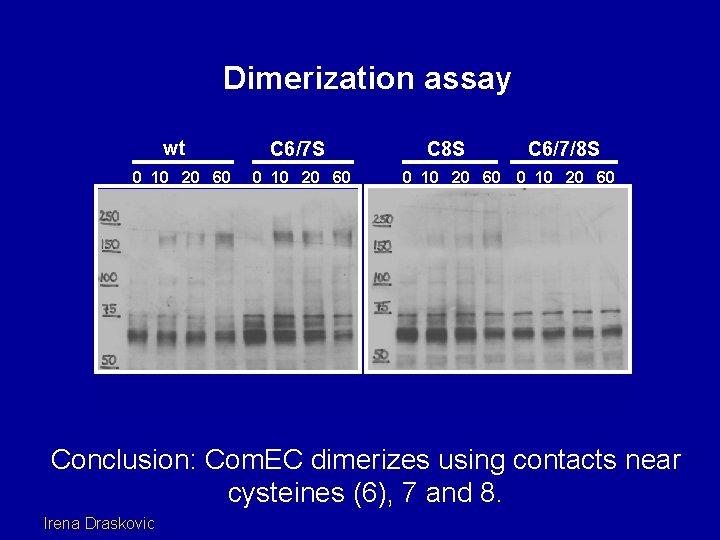
Dimerization assay wt 0 10 20 60 C 6/7 S 0 10 20 60 C 8 S 0 10 20 60 C 6/7/8 S 0 10 20 60 Conclusion: Com. EC dimerizes using contacts near cysteines (6), 7 and 8. Irena Draskovic
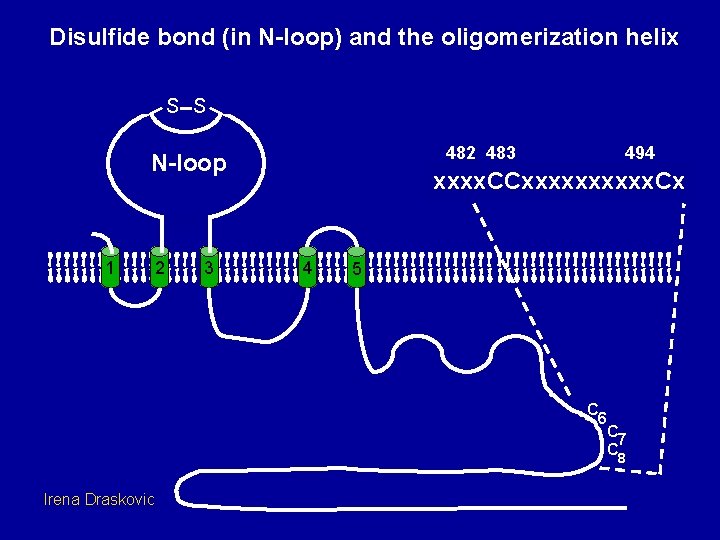
Disulfide bond (in N-loop) and the oligomerization helix S S 482 483 N-loop 1 2 3 494 xxxx. CCxxxxx. Cx 4 5 C 6 C 7 C 8 Irena Draskovic
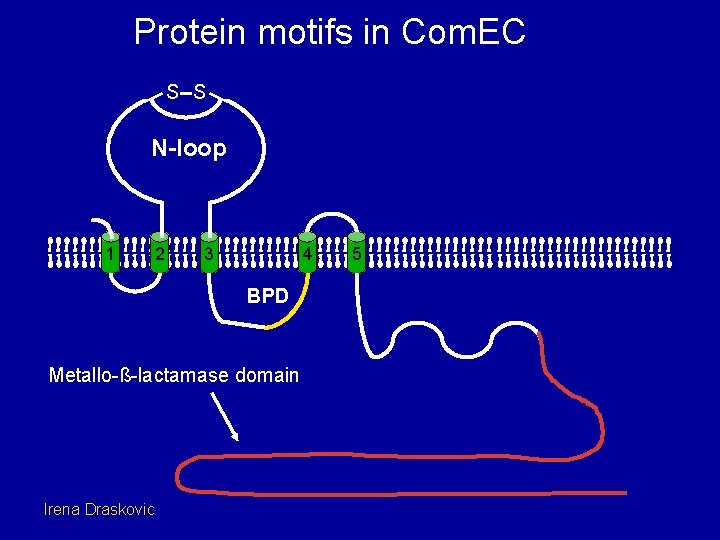
Protein motifs in Com. EC S S N-loop 1 2 3 4 BPD Metallo-ß-lactamase domain Irena Draskovic 5
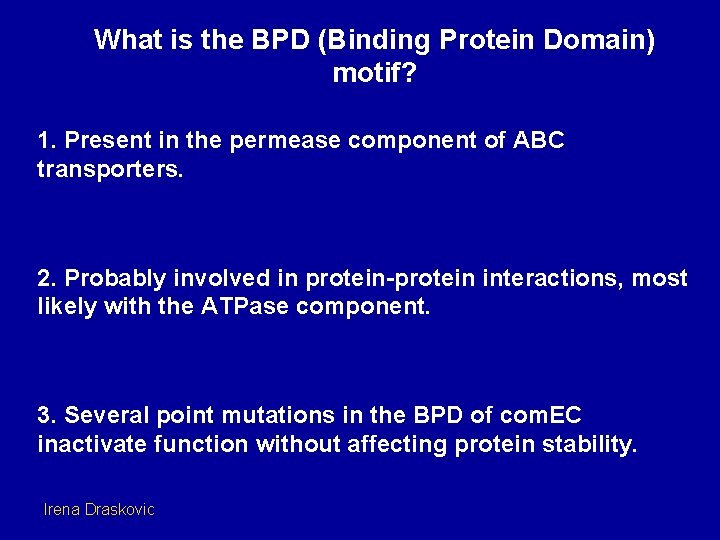
What is the BPD (Binding Protein Domain) motif? 1. Present in the permease component of ABC transporters. 2. Probably involved in protein-protein interactions, most likely with the ATPase component. 3. Several point mutations in the BPD of com. EC inactivate function without affecting protein stability. Irena Draskovic
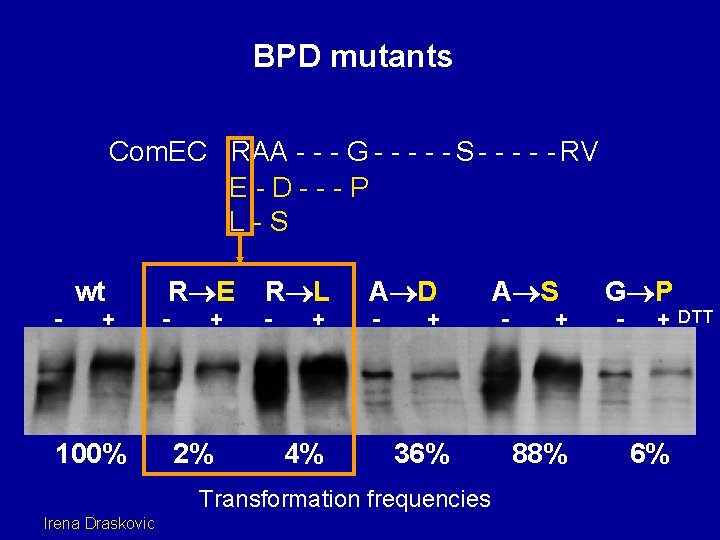
BPD mutants Com. EC RAA - - - G - - - S - - - RV E-D---P L-S - wt + 100% R E - + 2% R L - + 4% A D - + 36% Transformation frequencies Irena Draskovic A S - + 88% G P - + DTT 6%
What is a metallo-ß-lactamase domain? 1. Present in a large family of proteins. 2. Includes enzymes that use water for nucleophilic attacks on covalent bonds. Usually with dinuclear Zn(II) centers. 3. Substrates often esters with associated negative charges. 4. Artemis: Involved in V(D)J recombination and double strand break repair. Processively degrades ss. DNA (5’ 3’)!!! 5. Several point mutations, introduced in this domain of com. EC, lose function without affecting stability. 6. Possible roles in Com. EC: Cell-wall remodeling? Nontransforming strand nuclease? Irena Draskovic

ß-lactamase domain mutants Motif 1 Lh. Ds. G LIDTG N 2% Motif 4 Irena Draskovic hhxs. GD WILTGD Hx. DHx 2 HADQDHIG G A 3% C/H 3 A 4% 5 H A 4% hhxx. H KVGHH
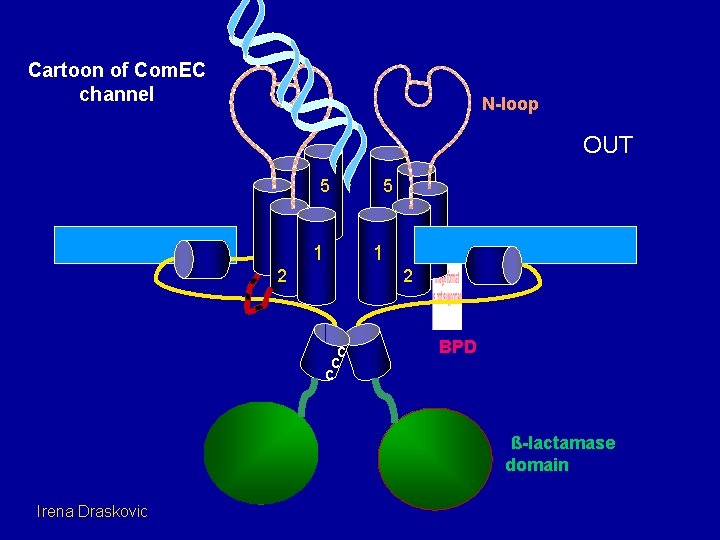
Cartoon of Com. EC channel N-loop OUT 5 5 4 3 1 2 2 c c c BPD ß-lactamase domain Irena Draskovic
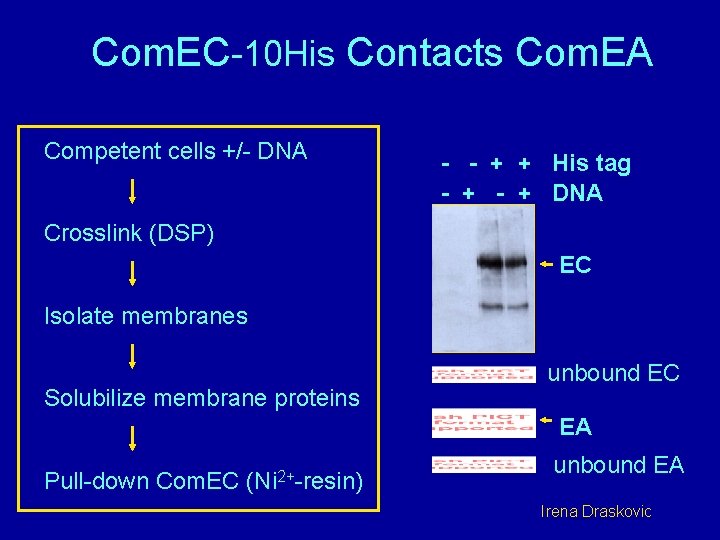
Com. EC-10 His Contacts Com. EA Competent cells +/- DNA - - + + His tag - + DNA Crosslink (DSP) EC Isolate membranes Solubilize membrane proteins unbound EC EA Pull-down Com. EC (Ni 2+-resin) unbound EA Irena Draskovic
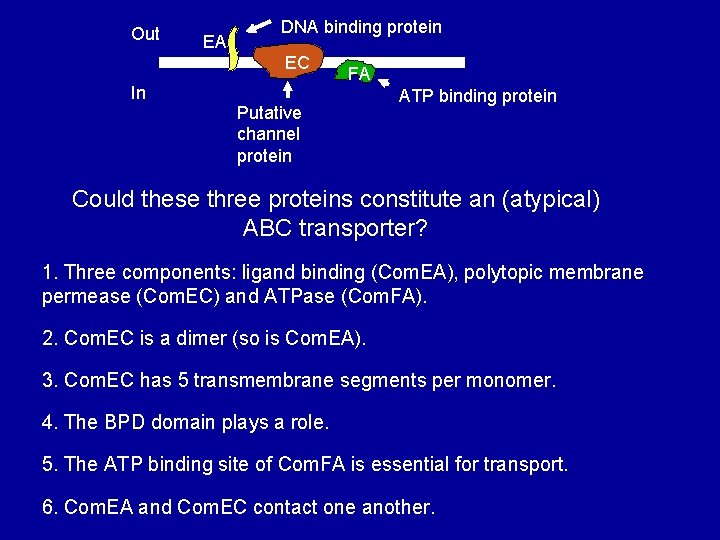
Out EA DNA binding protein EC In FA Putative channel protein ATP binding protein Could these three proteins constitute an (atypical) ABC transporter? 1. Three components: ligand binding (Com. EA), polytopic membrane permease (Com. EC) and ATPase (Com. FA). 2. Com. EC is a dimer (so is Com. EA). 3. Com. EC has 5 transmembrane segments per monomer. 4. The BPD domain plays a role. 5. The ATP binding site of Com. FA is essential for transport. 6. Com. EA and Com. EC contact one another.
But, transport may not be driven directly by ATP hydrolysis. Inhibitor data from the Konings lab (Groningen) suggests that transport is driven by the p. H component of PMF. Perhaps Com. FA plays a signaling role (channel gating? ) or is needed as a helicase, while PMF drives transport.
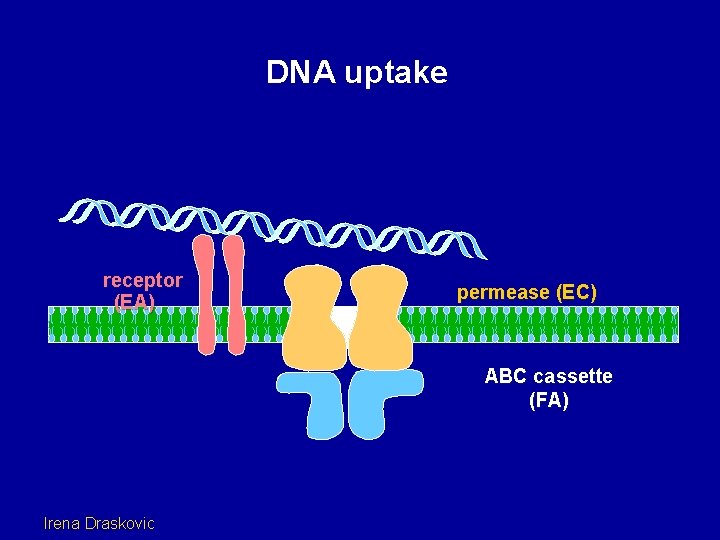
DNA uptake receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
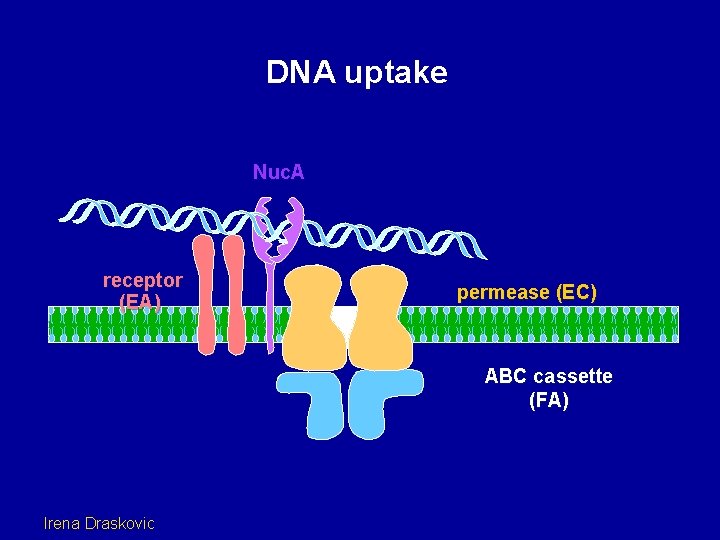
DNA uptake Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
DNA uptake Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
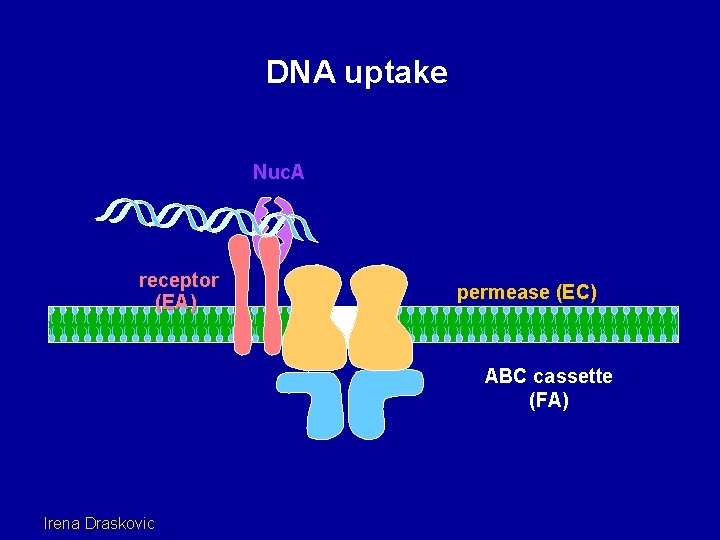
DNA uptake Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
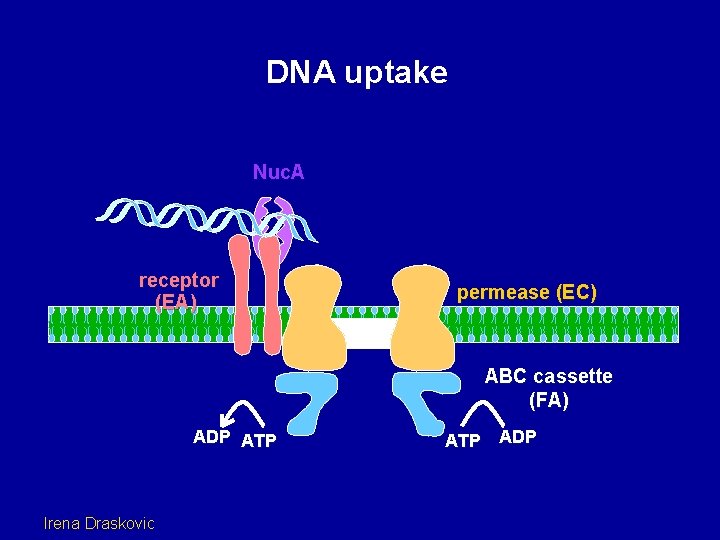
DNA uptake Nuc. A receptor (EA) permease (EC) ABC cassette (FA) ADP ATP Irena Draskovic ATP ADP
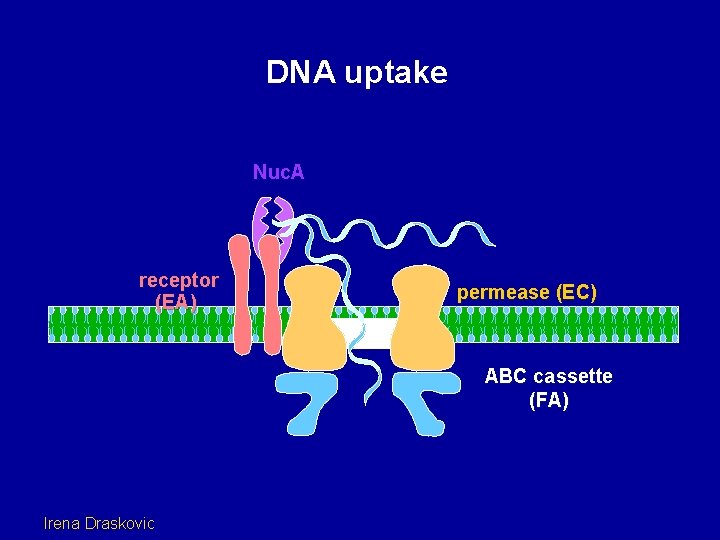
DNA uptake Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
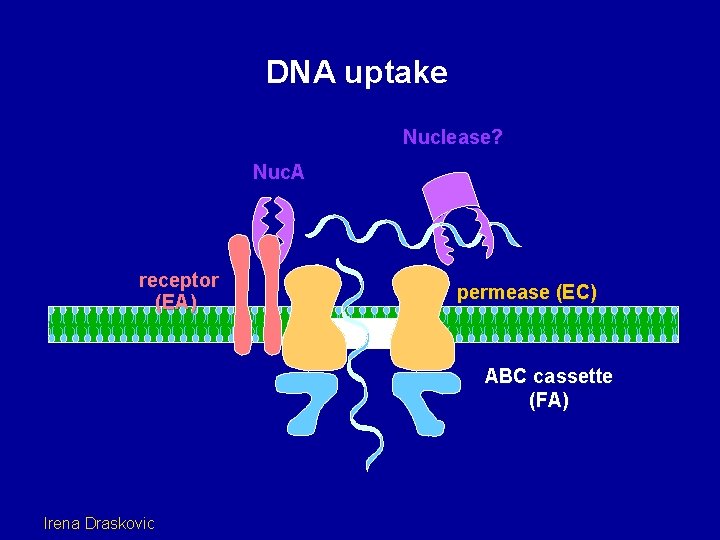
DNA uptake Nuclease? Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic
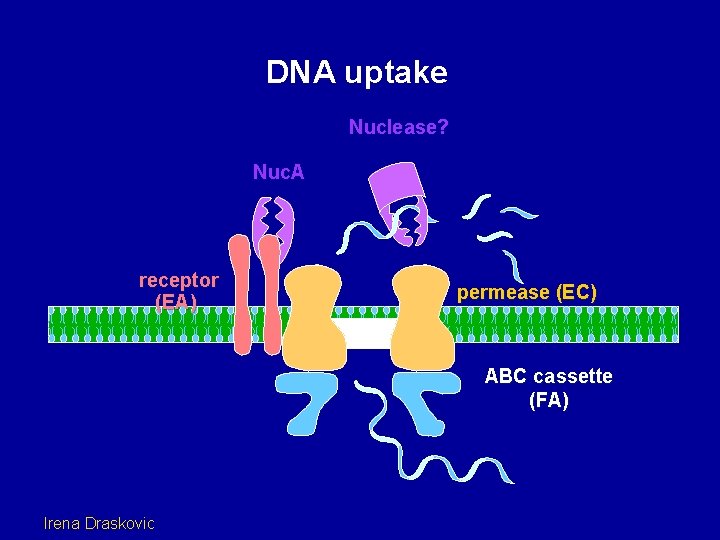
DNA uptake Nuclease? Nuc. A receptor (EA) permease (EC) ABC cassette (FA) Irena Draskovic

Transformation at the poles?
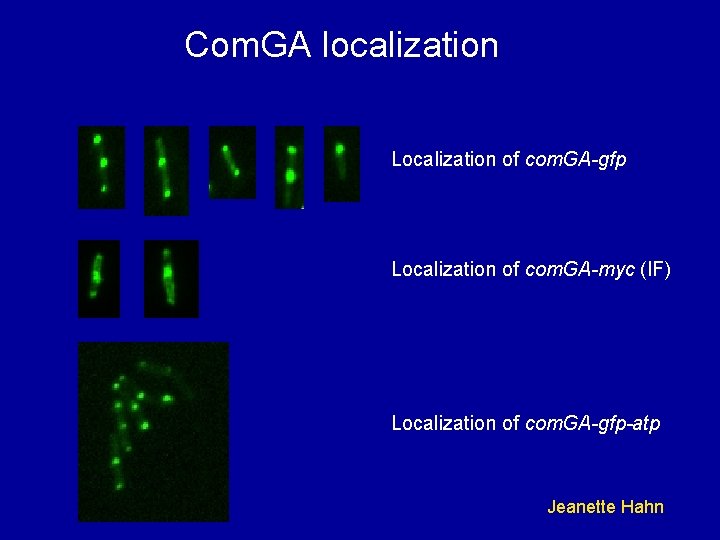
Com. GA localization Localization of com. GA-gfp Localization of com. GA-myc (IF) Localization of com. GA-gfp-atp Jeanette Hahn
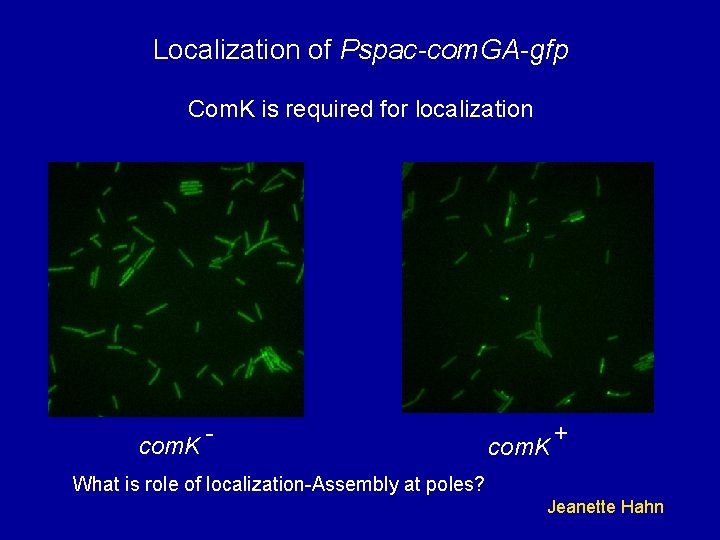
Localization of Pspac-com. GA-gfp Com. K is required for localization com. K - com. K + What is role of localization-Assembly at poles? Jeanette Hahn
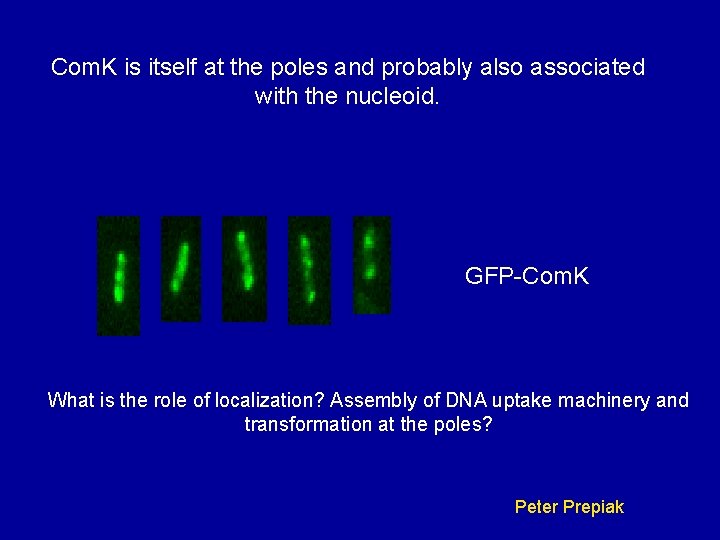
Com. K is itself at the poles and probably also associated with the nucleoid. GFP-Com. K What is the role of localization? Assembly of DNA uptake machinery and transformation at the poles? Peter Prepiak
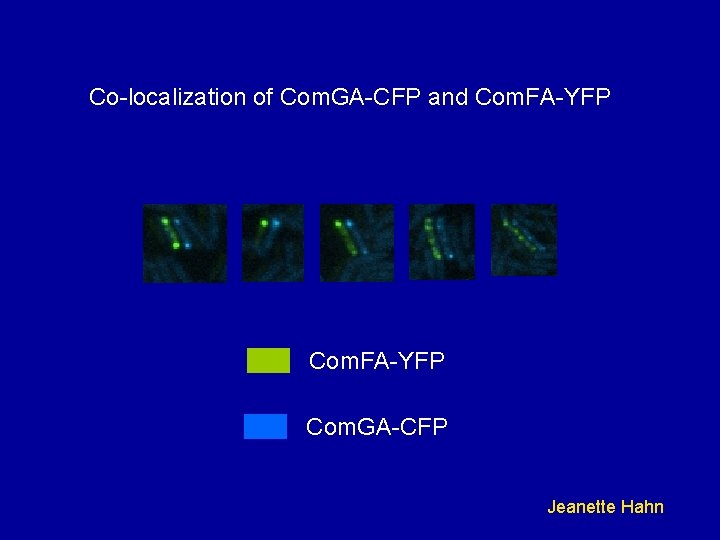
Co-localization of Com. GA-CFP and Com. FA-YFP Com. GA-CFP Jeanette Hahn
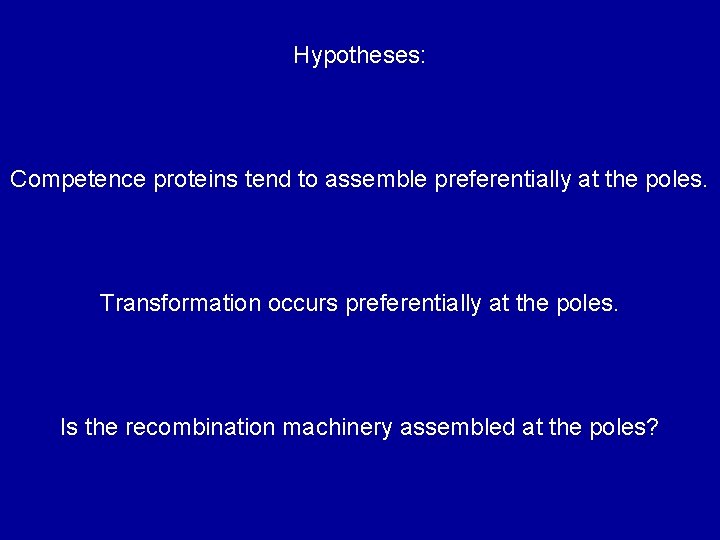
Hypotheses: Competence proteins tend to assemble preferentially at the poles. Transformation occurs preferentially at the poles. Is the recombination machinery assembled at the poles?
Regulation DNA uptake Mark Albano Mireille Ansaldi Mathieu Bergé Soo Jeong Cho Reinhard Breitling Jeanette Hahn Fred Breidt Leendert Hamoen Ines Chen Lauren Logsdon-Peterson Marjan Persuh Peter Prepiak Flavia Piazza Kursad Turgay Thi Yen Linh Ho Young Sook Chung Irena Draskovic Jeanette Hahn Gordon Inamine Arturo Londoño Sandhya Mohan Roberta Provvedi Pablo Tortosa Roopesh Kumar Pundhir Tran Thu Hoa Tatjana Trcek

Collaborations Bdb. DC: Sierd Bron et al, Groningen Optical trap: Berenike Meier and Michael Sheetz, Columbia University Action at the com. K promoter: Leendert Hamoen, Wiep Klaas Smits and Oscar Kuipers, Groningen

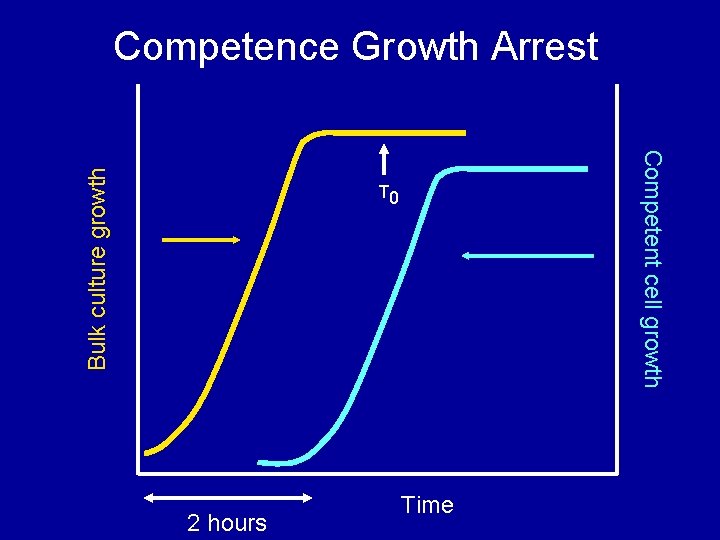
Competent cell growth Bulk culture growth Competence Growth Arrest T 0 2 hours Time
Com. EC channel N-loop 7 76 3 1 2 3 9 BPD 10 10 2 1 5 67 7 9 5 8 8 3 4 ß-lactamase domain
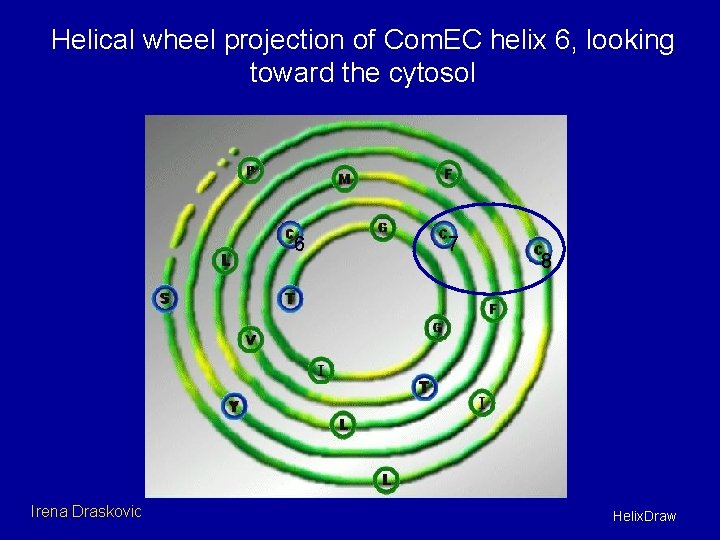
Helical wheel projection of Com. EC helix 6, looking toward the cytosol 6 Irena Draskovic 7 8 Helix. Draw
- Slides: 68