DNA Barcoding Workflow Image used with permission from
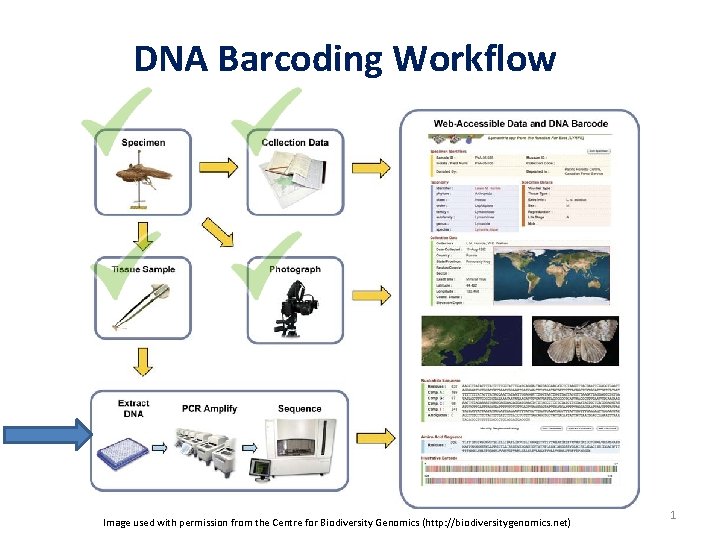
DNA Barcoding Workflow Image used with permission from the Centre for Biodiversity Genomics (http: //biodiversitygenomics. net) 1

To do DNA extractions you need: Skills: Vortexors Pipettors Microcentrifuge We’ll start with pipetting, after a reminder about units and conversions.
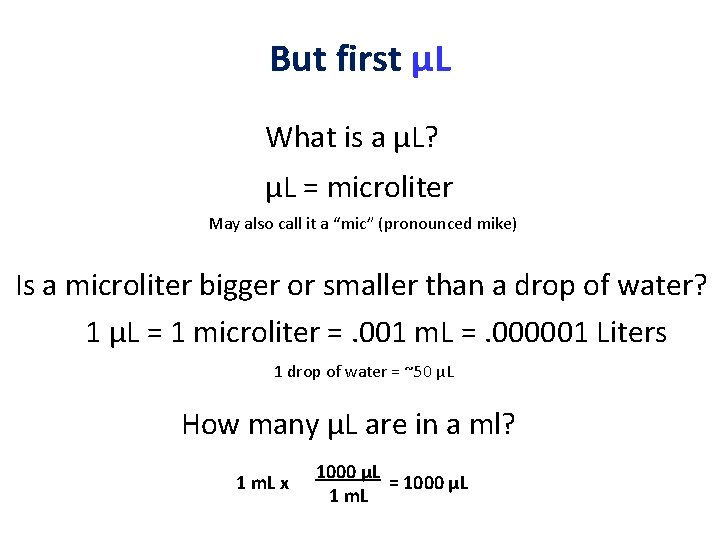
But first µL What is a µL? µL = microliter May also call it a “mic” (pronounced mike) Is a microliter bigger or smaller than a drop of water? 1 µL = 1 microliter =. 001 m. L =. 000001 Liters 1 drop of water = ~50 µL How many µL are in a ml? 1 m. L x 1000 µL = 1000 µL 1 m. L

Pipetting For a good overview of basic pipetting, please show: https: //www. youtube. com/watch? v=wc. Lfq. Kn. AE-k Hyman et al. CURE-all: DNA barcoding in introductory biology
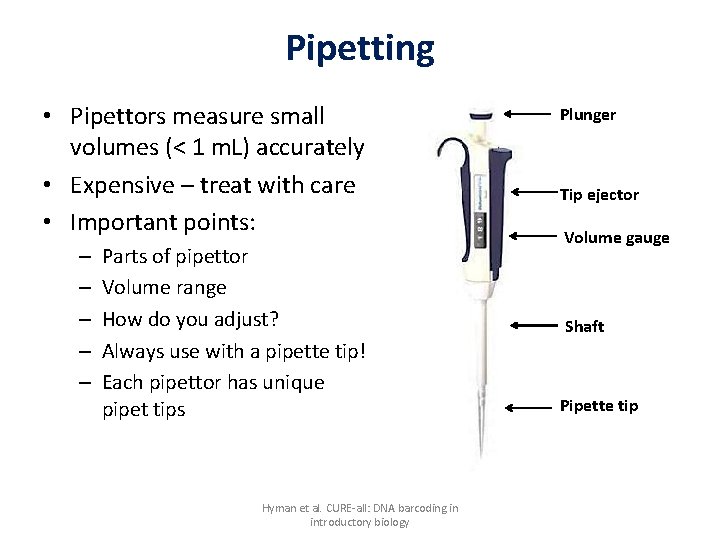
Pipetting • Pipettors measure small volumes (< 1 m. L) accurately • Expensive – treat with care • Important points: – – – Parts of pipettor Volume range How do you adjust? Always use with a pipette tip! Each pipettor has unique pipet tips Hyman et al. CURE-all: DNA barcoding in introductory biology Plunger Tip ejector Volume gauge Shaft Pipette tip

Pipetting – Things NOT to do! • NEVER dial a pipettor too high or too low! – Volume range is listed on the side of pipettor • NEVER use a pipettor without a pipette tip. • NEVER turn a pipettor upside down with liquid in the tip! • NEVER leave a pipettor on its side. For a funny comic on pipetting please visit: http: //www. biocomicals. com/ind_c omics. V 2. php? number=20120808 Remember, pipetting is a one person job – two hands on one body. Practice picking up and putting down tubes while you are pipetting!
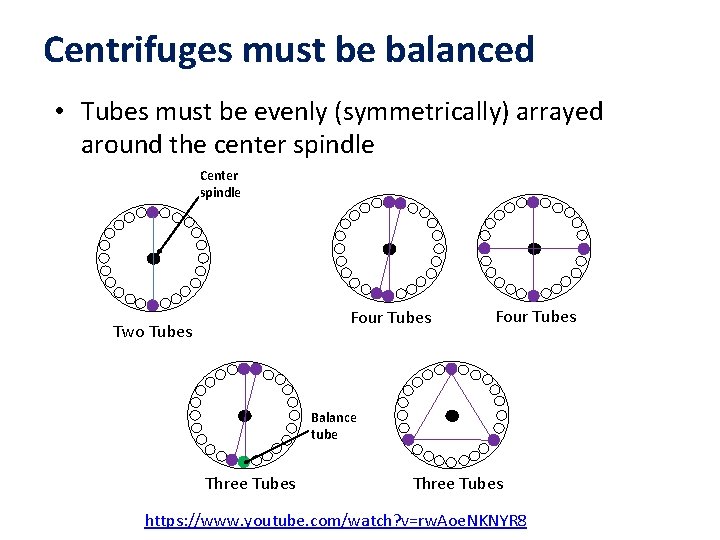
Centrifuges must be balanced • Tubes must be evenly (symmetrically) arrayed around the center spindle Center spindle Four Tubes Two Tubes Four Tubes Balance tube Three Tubes https: //www. youtube. com/watch? v=rw. Aoe. NKNYR 8
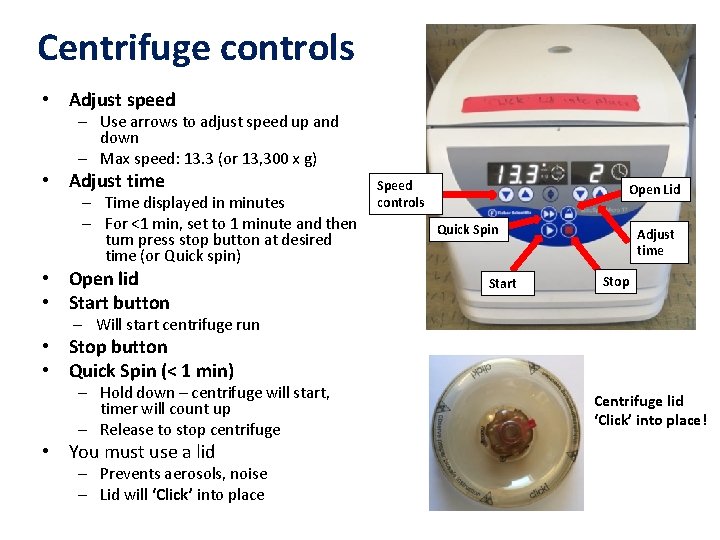
Centrifuge controls • Adjust speed – Use arrows to adjust speed up and down – Max speed: 13. 3 (or 13, 300 x g) • Adjust time – Time displayed in minutes – For <1 min, set to 1 minute and then turn press stop button at desired time (or Quick spin) • Open lid • Start button Speed controls Open Lid Quick Spin Start Adjust time Stop – Will start centrifuge run • Stop button • Quick Spin (< 1 min) – Hold down – centrifuge will start, timer will count up – Release to stop centrifuge • You must use a lid – Prevents aerosols, noise – Lid will ‘Click’ into place Centrifuge lid ‘Click’ into place!
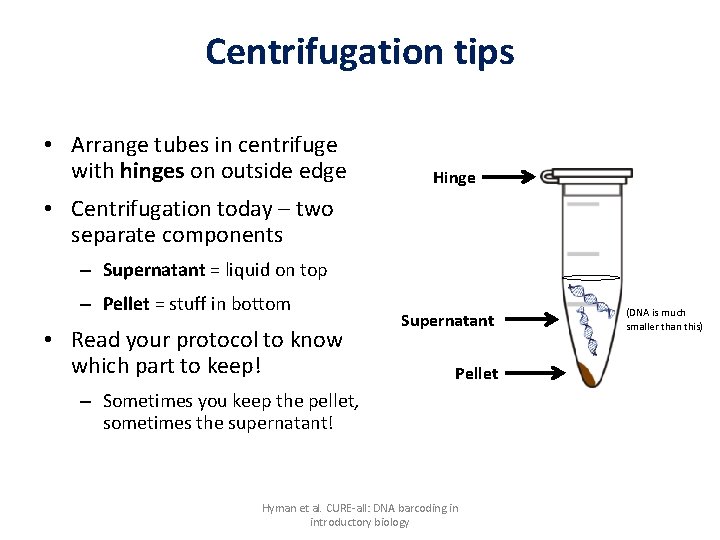
Centrifugation tips • Arrange tubes in centrifuge with hinges on outside edge Hinge • Centrifugation today – two separate components – Supernatant = liquid on top – Pellet = stuff in bottom • Read your protocol to know which part to keep! Supernatant Pellet – Sometimes you keep the pellet, sometimes the supernatant! Hyman et al. CURE-all: DNA barcoding in introductory biology (DNA is much smaller than this)
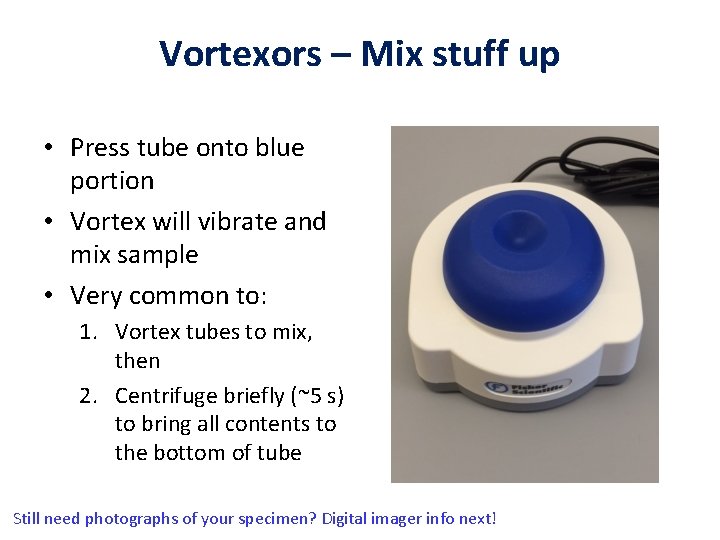
Vortexors – Mix stuff up • Press tube onto blue portion • Vortex will vibrate and mix sample • Very common to: 1. Vortex tubes to mix, then 2. Centrifuge briefly (~5 s) to bring all contents to the bottom of tube Still need photographs of your specimen? Digital imager info next!

First Lab exercises – let’s get started • Work individually for all exercises • Pipetting Exercises First – Post-rinsing of small volumes – very important! – Always use the smallest pipettor for a volume • Pipettors are most accurate in their middle to upper range. – Perform the pipetting exercises – start at different places/exercises in the manual! • Centrifugation – Centrifuging silica resin – Vortexors • Avoiding contamination – no exercises, just common sense Remember your safety glasses!! Never leave pipette tip boxes open!!

DNA Extraction DNA is inside cells - must be extracted We recommend an image from Chromosomes, DNA and Genes: http: //www. bbc. co. uk/education/guides/z p 7 thyc/revision/2 Hyman et al. CURE-all: DNA barcoding in introductory biology
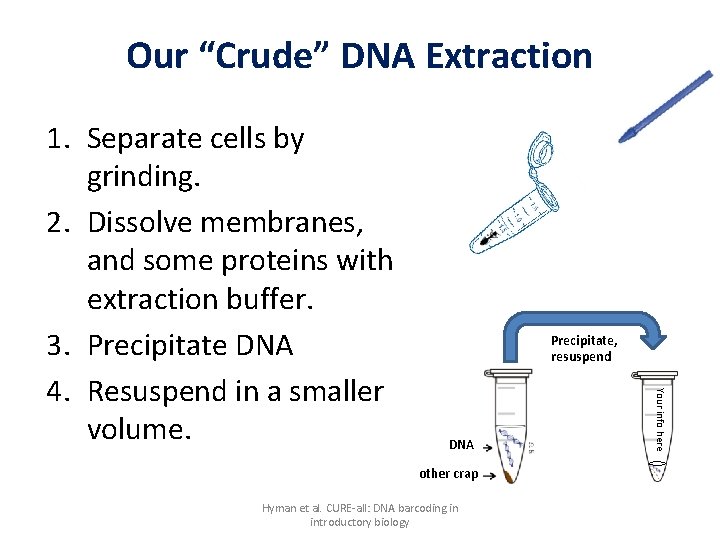
Our “Crude” DNA Extraction Precipitate, resuspend DNA other crap Hyman et al. CURE-all: DNA barcoding in introductory biology Your info here 1. Separate cells by grinding. 2. Dissolve membranes, and some proteins with extraction buffer. 3. Precipitate DNA 4. Resuspend in a smaller volume.

Tips: DNA is everywhere, Contamination is Easy For a humorous DNA testing image, please visit: http: //www. lemonharanguepie. com/2013/01/ https: //www. youtube. com/watch? v=xxndtk. XXBh. E for a video on how barrier pipet tips work.

Preventing contamination • Wipe down your bench with cleaner • Wear gloves. – Change gloves if you suspect yours have been contaminated • Open and close all sample tubes and reaction plates carefully. • Keep test tubes and pipette tip boxes closed • Change pipette tips if tips will be touching things that will touch other samples • Clean scissors and other tools before and after using them Hyman et al. CURE-all: DNA barcoding in introductory biology
Hints • Clean bench, Goggles on, Gloves on • Add about ¼ - ½ pencil-eraser size of tissue – Avoid stomachs of large insects • Read the protocol carefully – use as a checklist. • Put used pestle in beaker on side bench. • Toss used vials in “used tips” container on bench – Don’t toss colored liquid vials • When all done, label your tube with colored tape and bring to me Hyman et al. CURE-all: DNA barcoding in introductory biology CM Inches
- Slides: 16