DNA Amplification Research Technology Development Diagnostics Group PMC









- Slides: 48

DNA Amplification Research & Technology Development Diagnostics Group, PMC Advanced Technology

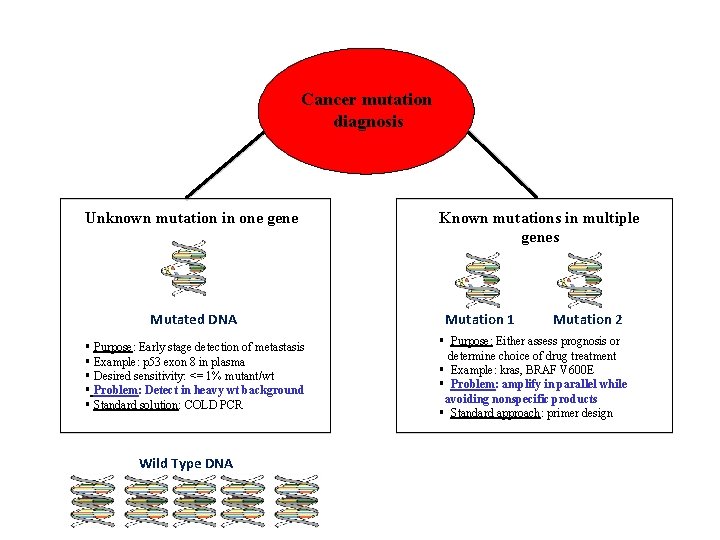
Cancer mutation diagnosis Unknown mutation in one gene Mutated DNA § Purpose: Early stage detection of metastasis § Example: p 53 exon 8 in plasma § Desired sensitivity: <= 1% mutant/wt § Problem: Detect in heavy wt background § Standard solution: COLD PCR Wild Type DNA Known mutations in multiple genes Mutation 1 Mutation 2 § Purpose: Either assess prognosis or determine choice of drug treatment § Example: kras, BRAF V 600 E § Problem: amplify in parallel while avoiding nonspecific products § Standard approach: primer design

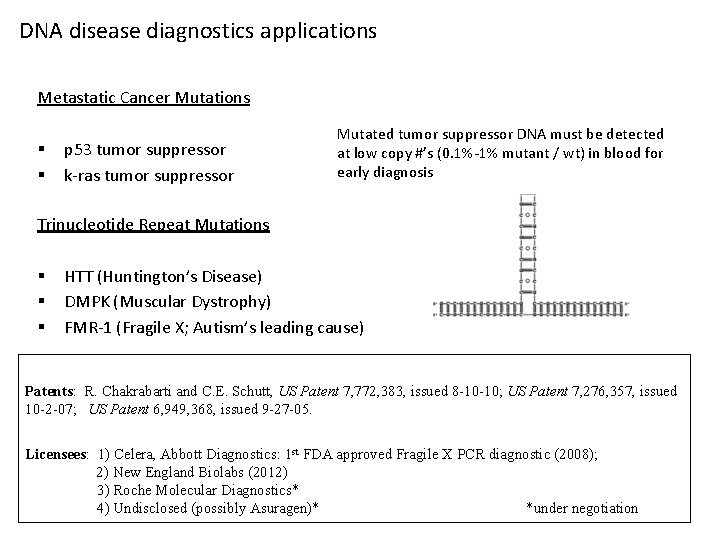
DNA disease diagnostics applications Metastatic Cancer Mutations § § p 53 tumor suppressor k-ras tumor suppressor Mutated tumor suppressor DNA must be detected at low copy #’s (0. 1%-1% mutant / wt) in blood for early diagnosis Trinucleotide Repeat Mutations § § § HTT (Huntington’s Disease) DMPK (Muscular Dystrophy) FMR-1 (Fragile X; Autism’s leading cause) Patents: R. Chakrabarti and C. E. Schutt, US Patent 7, 772, 383, issued 8 -10 -10; US Patent 7, 276, 357, issued 10 -2 -07; US Patent 6, 949, 368, issued 9 -27 -05. Licensees: 1) Celera, Abbott Diagnostics: 1 st FDA approved Fragile X PCR diagnostic (2008); 2) New England Biolabs (2012) 3) Roche Molecular Diagnostics* 4) Undisclosed (possibly Asuragen)* *under negotiation

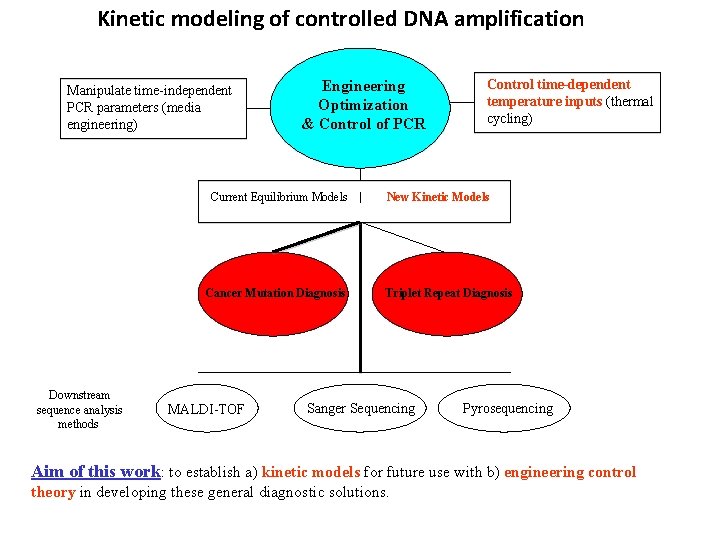
Kinetic modeling of controlled DNA amplification Manipulate time-independent PCR parameters (media engineering) Engineering Optimization & Control of PCR Control time-dependent temperature inputs (thermal cycling) Current Equilibrium Models | New Kinetic Models Cancer Mutation Diagnosis Downstream sequence analysis methods MALDI-TOF Triplet Repeat Diagnosis Sanger Sequencing Pyrosequencing Aim of this work: to establish a) kinetic models for future use with b) engineering control theory in developing these general diagnostic solutions.

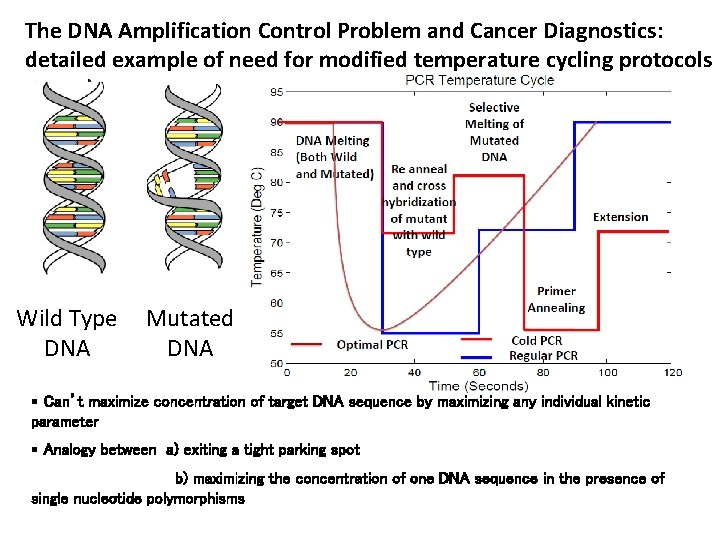
The DNA Amplification Control Problem and Cancer Diagnostics: detailed example of need for modified temperature cycling protocols Wild Type DNA Mutated DNA § Can’t maximize concentration of target DNA sequence by maximizing any individual kinetic parameter § Analogy between a) exiting a tight parking spot b) maximizing the concentration of one DNA sequence in the presence of single nucleotide polymorphisms

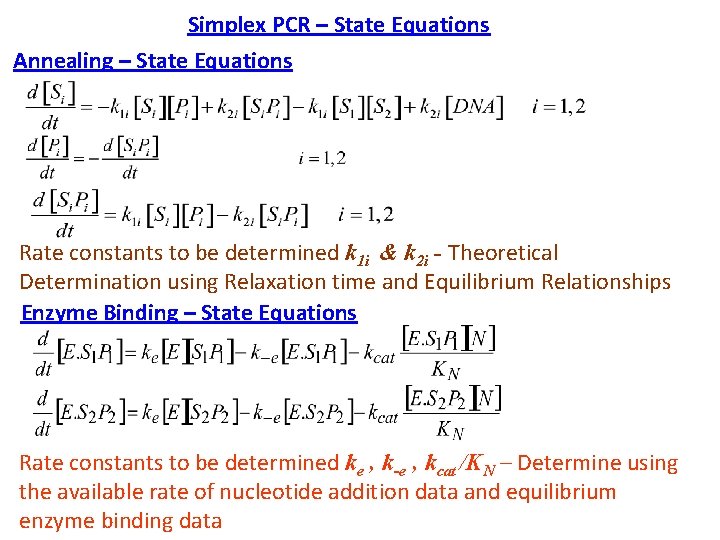
Simplex PCR – State Equations Annealing – State Equations Rate constants to be determined k 1 i & k 2 i - Theoretical Determination using Relaxation time and Equilibrium Relationships Enzyme Binding – State Equations Rate constants to be determined ke , k-e , kcat /KN – Determine using the available rate of nucleotide addition data and equilibrium enzyme binding data

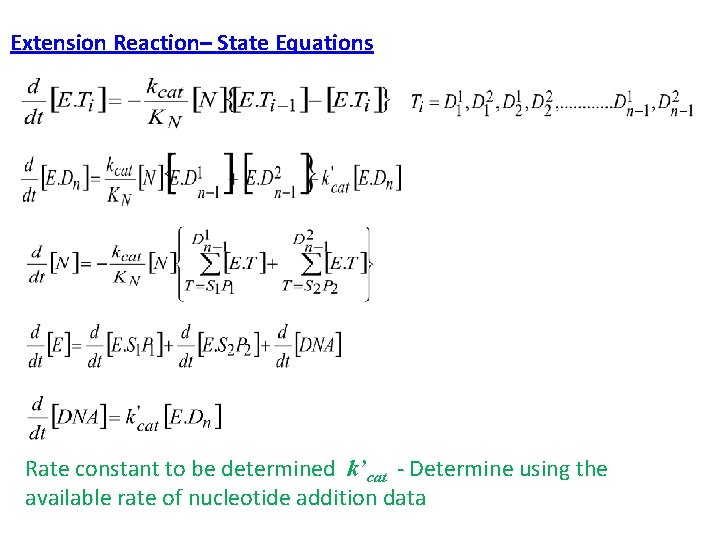
Extension Reaction– State Equations Rate constant to be determined k’cat - Determine using the available rate of nucleotide addition data

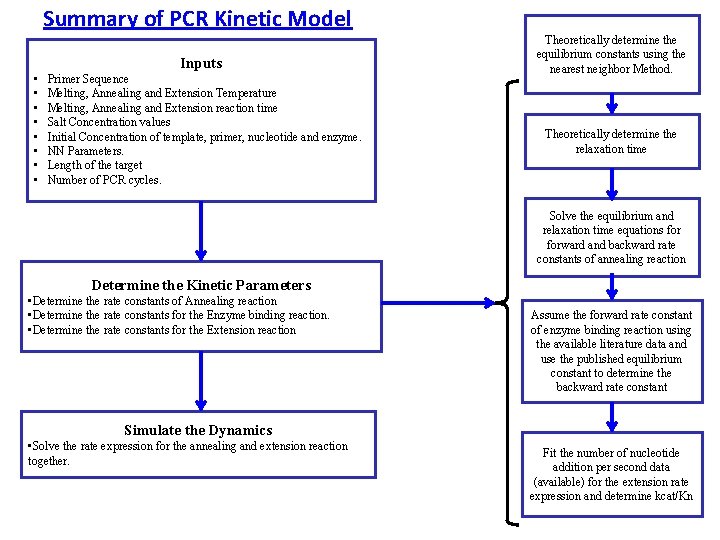
Summary of PCR Kinetic Model Inputs • • Primer Sequence Melting, Annealing and Extension Temperature Melting, Annealing and Extension reaction time Salt Concentration values Initial Concentration of template, primer, nucleotide and enzyme. NN Parameters. Length of the target Number of PCR cycles. Theoretically determine the equilibrium constants using the nearest neighbor Method. Theoretically determine the relaxation time Solve the equilibrium and relaxation time equations forward and backward rate constants of annealing reaction Determine the Kinetic Parameters • Determine the rate constants of Annealing reaction • Determine the rate constants for the Enzyme binding reaction. • Determine the rate constants for the Extension reaction Assume the forward rate constant of enzyme binding reaction using the available literature data and use the published equilibrium constant to determine the backward rate constant Simulate the Dynamics • Solve the rate expression for the annealing and extension reaction together. Fit the number of nucleotide addition per second data (available) for the extension rate expression and determine kcat/Kn

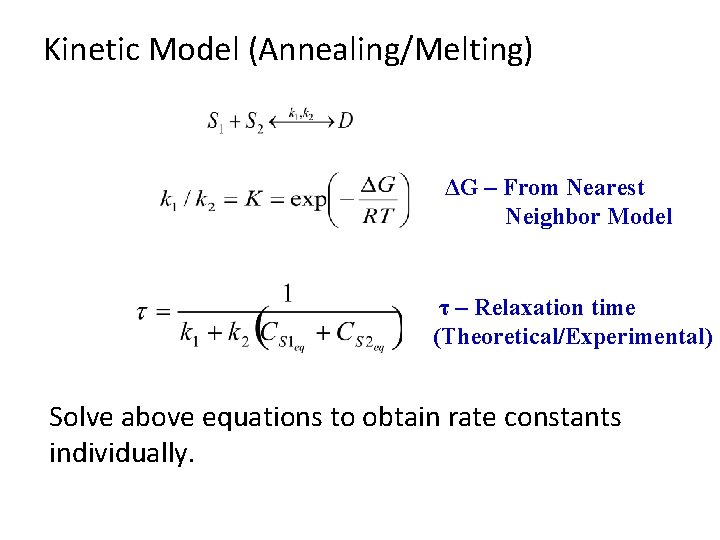
Kinetic Model (Annealing/Melting) ΔG – From Nearest Neighbor Model τ – Relaxation time (Theoretical/Experimental) Solve above equations to obtain rate constants individually.

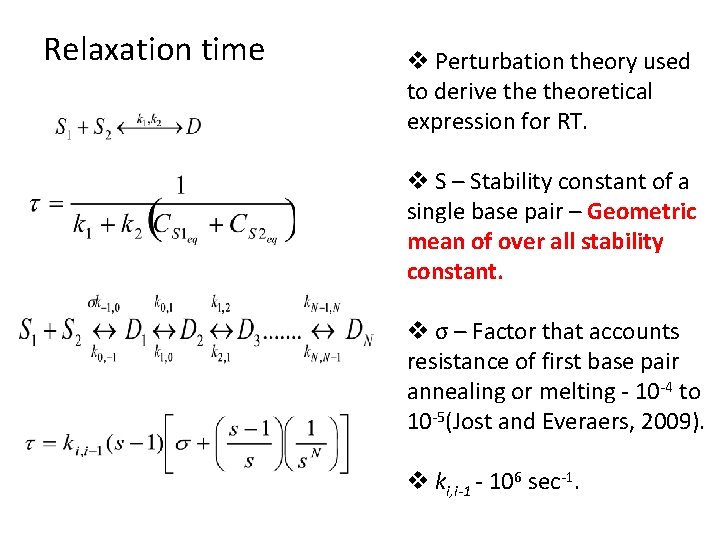
Relaxation time v Perturbation theory used to derive theoretical expression for RT. v S – Stability constant of a single base pair – Geometric mean of over all stability constant. v σ – Factor that accounts resistance of first base pair annealing or melting - 10 -4 to 10 -5(Jost and Everaers, 2009). v ki, i-1 - 106 sec-1.

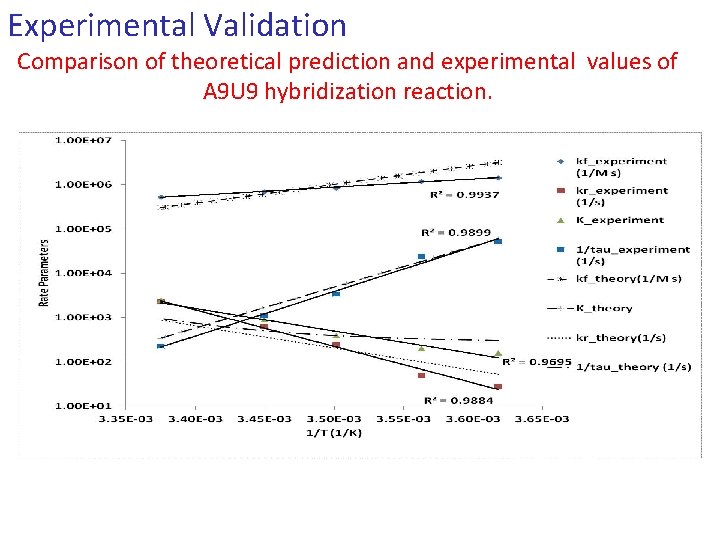
Experimental Validation Comparison of theoretical prediction and experimental values of A 9 U 9 hybridization reaction.

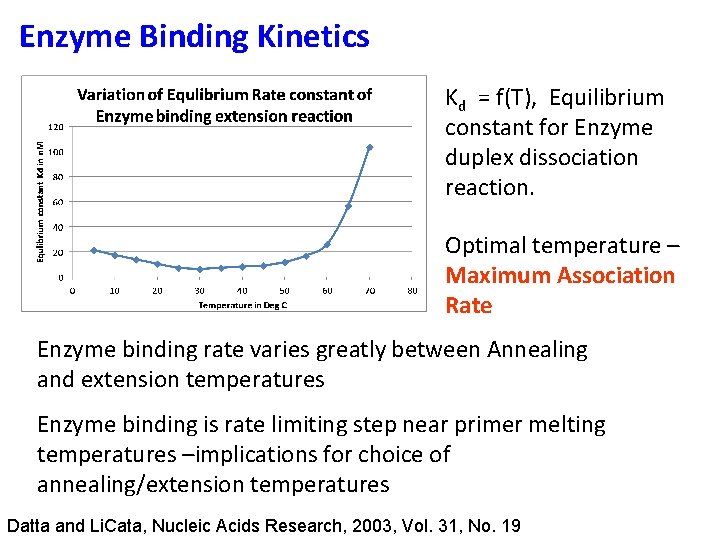
Enzyme Binding Kinetics Kd = f(T), Equilibrium constant for Enzyme duplex dissociation reaction. Optimal temperature – Maximum Association Rate Enzyme binding rate varies greatly between Annealing and extension temperatures Enzyme binding is rate limiting step near primer melting temperatures –implications for choice of annealing/extension temperatures Datta and Li. Cata, Nucleic Acids Research, 2003, Vol. 31, No. 19

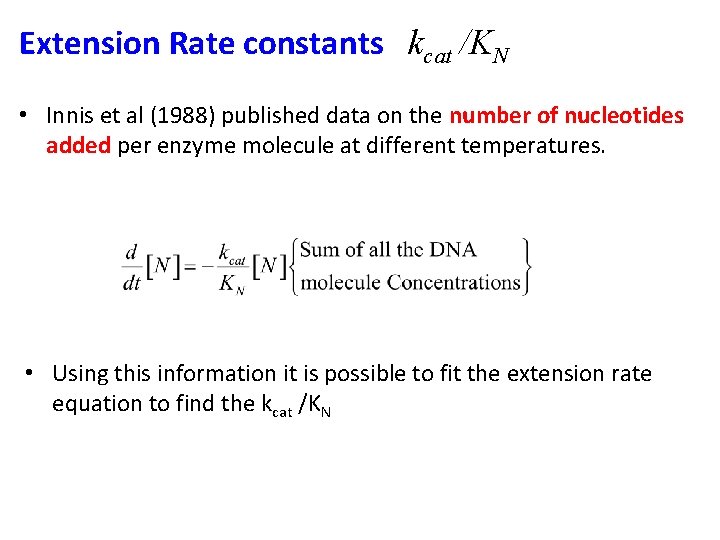
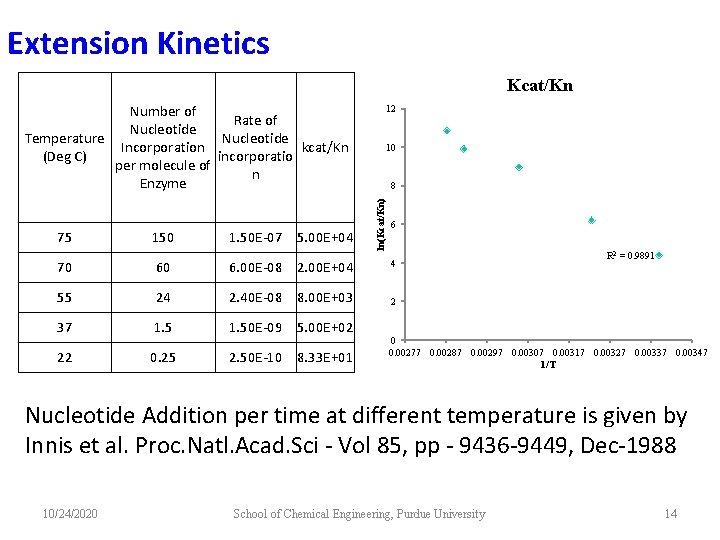
Extension Rate constants kcat /KN • Innis et al (1988) published data on the number of nucleotides added per enzyme molecule at different temperatures. • Using this information it is possible to fit the extension rate equation to find the kcat /KN

Extension Kinetics Kcat/Kn Number of Rate of Nucleotide Temperature Nucleotide Incorporation kcat/Kn (Deg C) incorporatio per molecule of n Enzyme 150 1. 50 E-07 5. 00 E+04 10 8 ln(Kcat/Kn) 75 12 6 70 60 6. 00 E-08 2. 00 E+04 4 55 24 2. 40 E-08 8. 00 E+03 2 37 1. 50 E-09 5. 00 E+02 22 0. 25 2. 50 E-10 8. 33 E+01 R 2 = 0. 9891 0 0. 00277 0. 00287 0. 00297 0. 00307 0. 00317 0. 00327 0. 00337 0. 00347 1/T Nucleotide Addition per time at different temperature is given by Innis et al. Proc. Natl. Acad. Sci - Vol 85, pp - 9436 -9449, Dec-1988 10/24/2020 School of Chemical Engineering, Purdue University 14

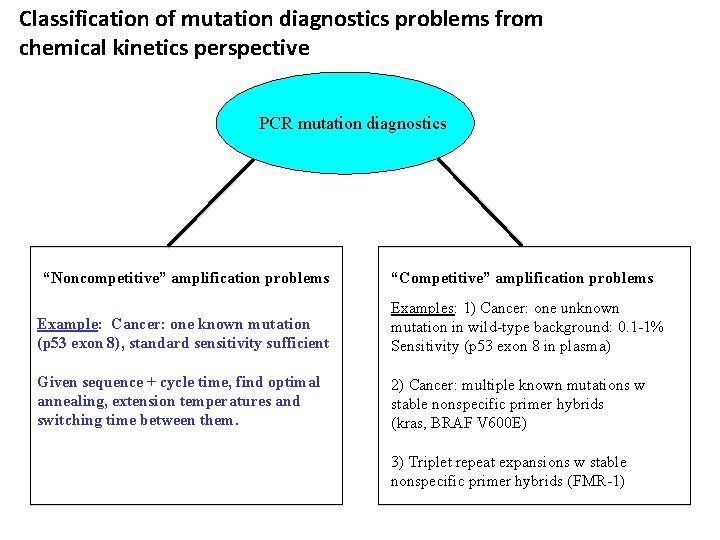
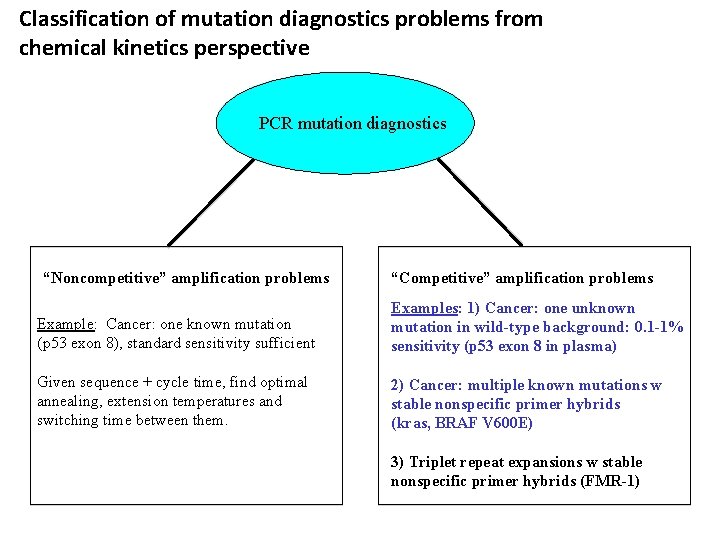
Classification of mutation diagnostics problems from chemical kinetics perspective PCR mutation diagnostics “Noncompetitive” amplification problems “Competitive” amplification problems Example: Cancer: one known mutation (p 53 exon 8), standard sensitivity sufficient Examples: 1) Cancer: one unknown mutation in wild-type background: 0. 1 -1% Sensitivity (p 53 exon 8 in plasma) Given sequence + cycle time, find optimal annealing, extension temperatures and switching time between them. 2) Cancer: multiple known mutations w stable nonspecific primer hybrids (kras, BRAF V 600 E) 3) Triplet repeat expansions w stable nonspecific primer hybrids (FMR-1)

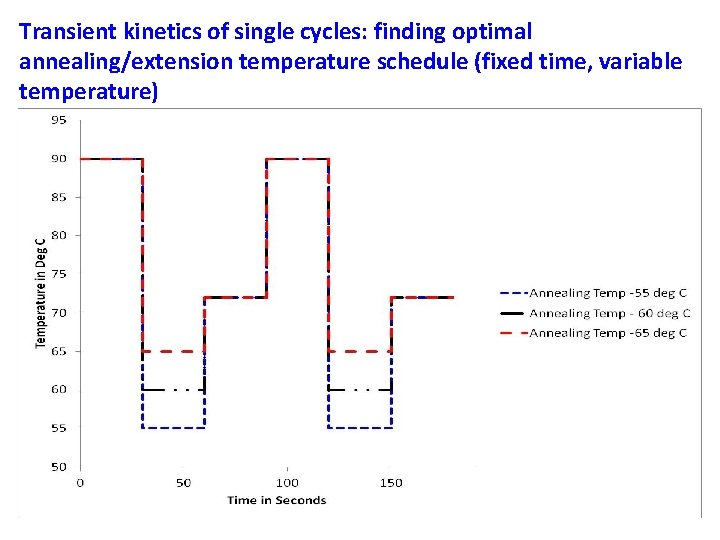
Transient kinetics of single cycles: finding optimal annealing/extension temperature schedule (fixed time, variable temperature) Annealing time – 30 sec

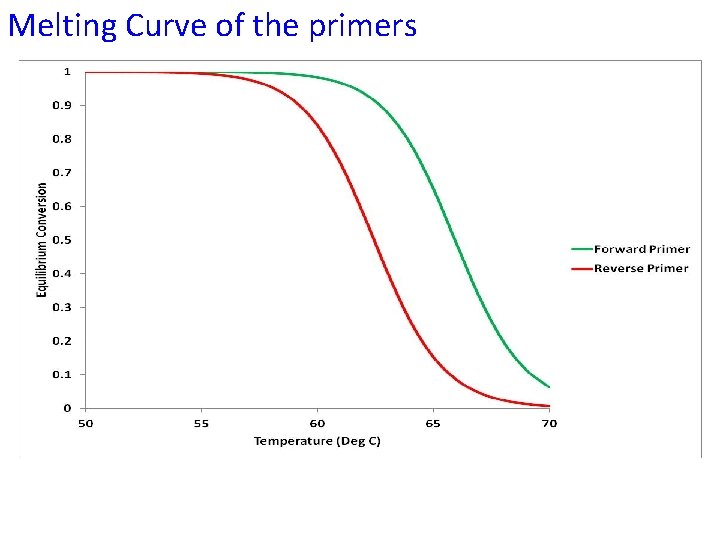
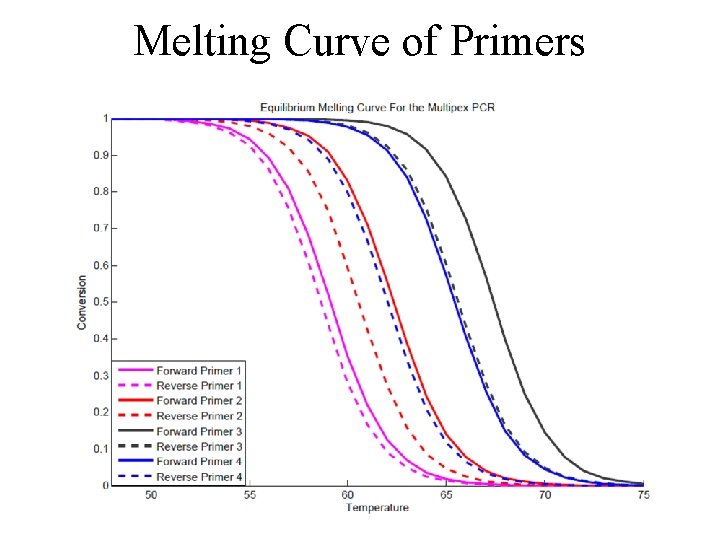
Melting Curve of the primers

Case 1 Length of the target = 480, Initial Concentration of the DNA during the start of the cycle = 2× 10 -14 M

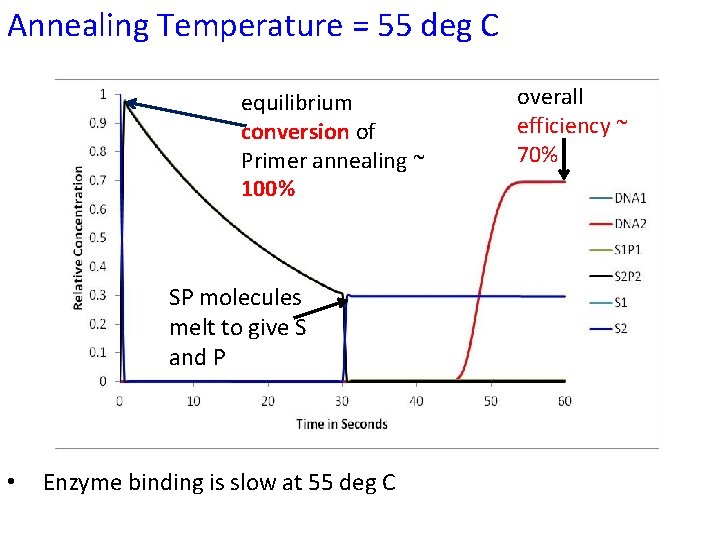
Annealing Temperature = 55 deg C equilibrium conversion of Primer annealing ~ 100% SP molecules melt to give S and P • Enzyme binding is slow at 55 deg C overall efficiency ~ 70%

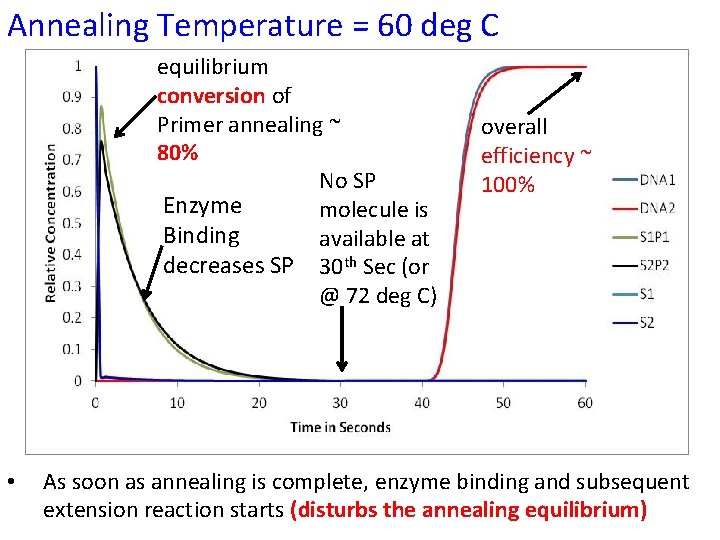
Annealing Temperature = 60 deg C equilibrium conversion of Primer annealing ~ 80% No SP Enzyme molecule is Binding available at decreases SP 30 th Sec (or @ 72 deg C) • overall efficiency ~ 100% As soon as annealing is complete, enzyme binding and subsequent extension reaction starts (disturbs the annealing equilibrium)

Case 2 Length of the target = 480, Initial Concentration of the DNA during the start of the cycle = 2× 10 -8 M

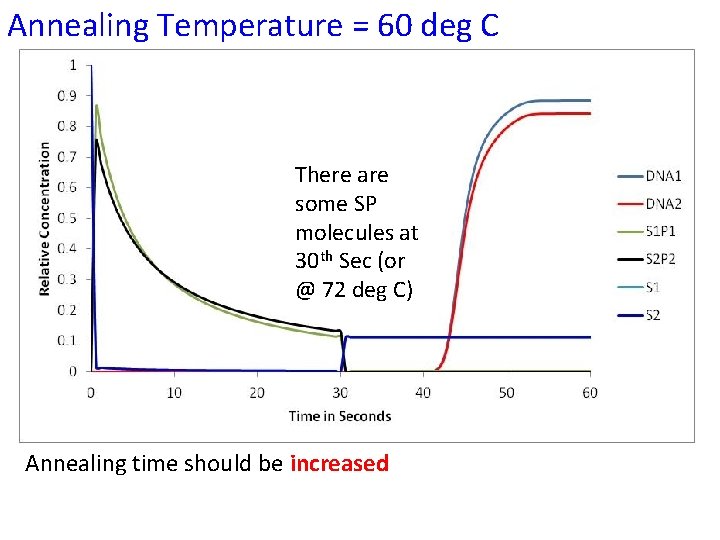
Annealing Temperature = 60 deg C There are some SP molecules at 30 th Sec (or @ 72 deg C) Annealing time should be increased

Summary • During the PCR, P/S ratio decreases and hence, the kinetics of Annealing reaction also changes. • When concentration of the template increases, Annealing and extension time need to be changes. • There is an optimal temperature at which reaction is quick and reaches 100% efficiency. • These observation can be formulated as an Optimal Control problem to find optimal time and temperature trajectory for a given template amplification.

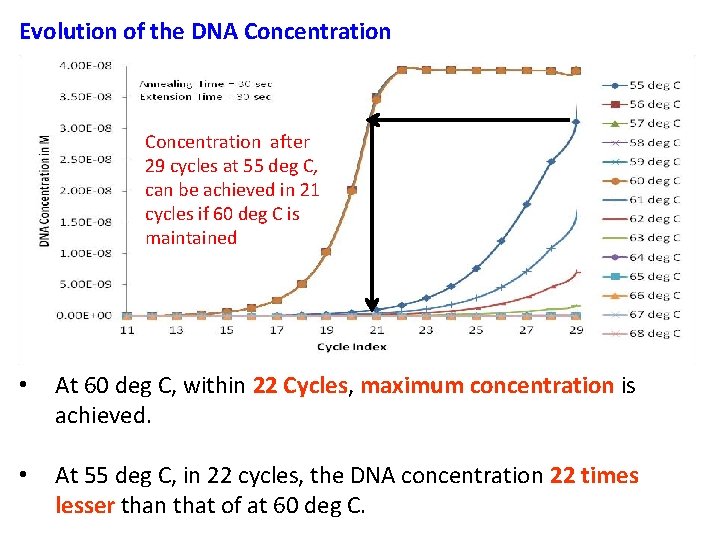
Evolution of the DNA Concentration after 29 cycles at 55 deg C, can be achieved in 21 cycles if 60 deg C is maintained • At 60 deg C, within 22 Cycles, maximum concentration is achieved. • At 55 deg C, in 22 cycles, the DNA concentration 22 times lesser than that of at 60 deg C.

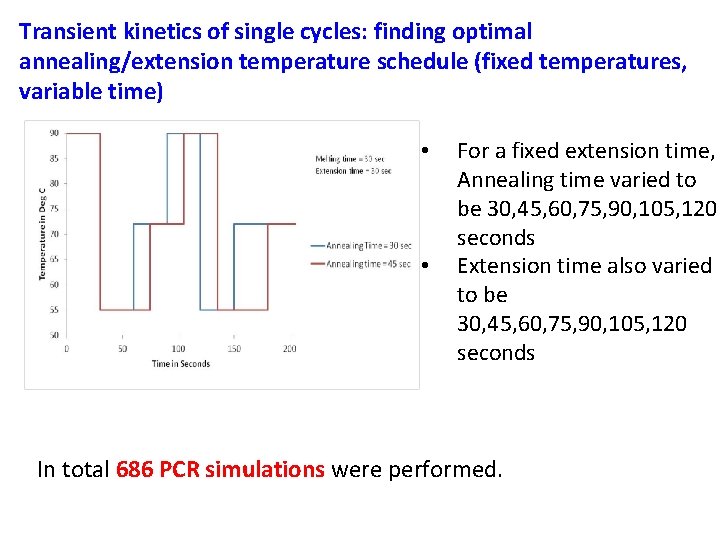
Transient kinetics of single cycles: finding optimal annealing/extension temperature schedule (fixed temperatures, variable time) • • For a fixed extension time, Annealing time varied to be 30, 45, 60, 75, 90, 105, 120 seconds Extension time also varied to be 30, 45, 60, 75, 90, 105, 120 seconds In total 686 PCR simulations were performed.

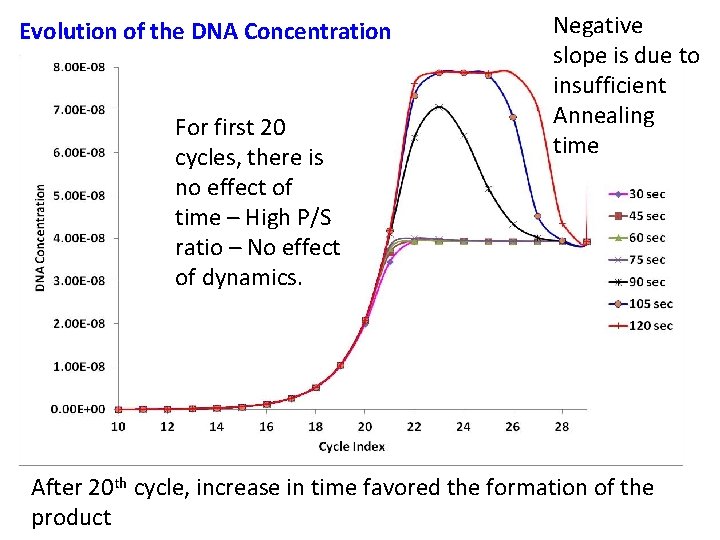
Evolution of the DNA Concentration For first 20 cycles, there is no effect of time – High P/S ratio – No effect of dynamics. Negative slope is due to insufficient Annealing time After 20 th cycle, increase in time favored the formation of the product

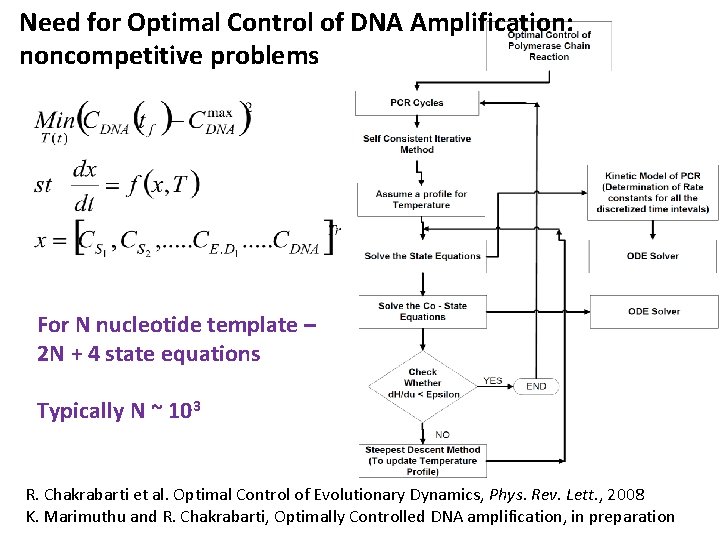
Need for Optimal Control of DNA Amplification: noncompetitive problems For N nucleotide template – 2 N + 4 state equations Typically N ~ 103 R. Chakrabarti et al. Optimal Control of Evolutionary Dynamics, Phys. Rev. Lett. , 2008 K. Marimuthu and R. Chakrabarti, Optimally Controlled DNA amplification, in preparation

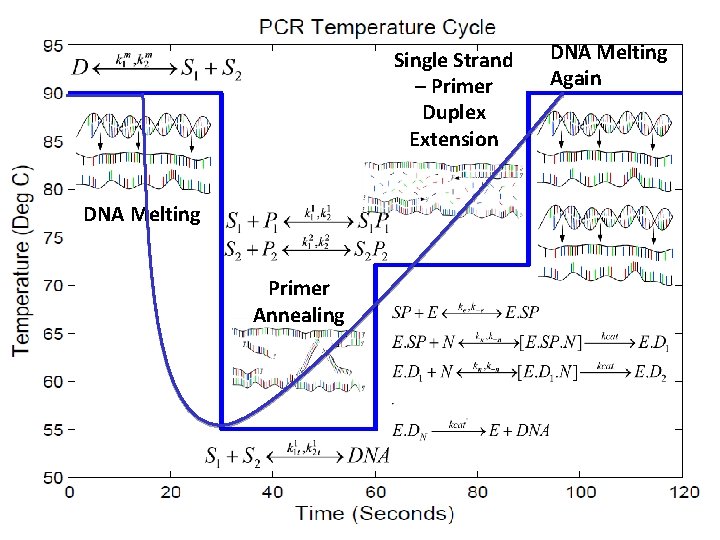
Single Strand – Primer Duplex Extension DNA Melting Again DNA Melting Primer Annealing 10/24/2020 School of Chemical Engineering, Purdue University 28

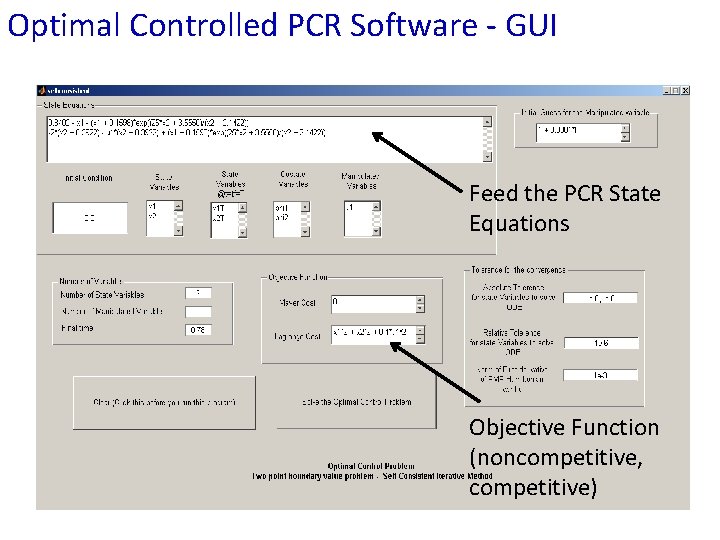
Optimal Controlled PCR Software - GUI Feed the PCR State Equations Objective Function (noncompetitive, competitive)

Classification of mutation diagnostics problems from chemical kinetics perspective PCR mutation diagnostics “Noncompetitive” amplification problems “Competitive” amplification problems Example: Cancer: one known mutation (p 53 exon 8), standard sensitivity sufficient Examples: 1) Cancer: one unknown mutation in wild-type background: 0. 1 -1% sensitivity (p 53 exon 8 in plasma) Given sequence + cycle time, find optimal annealing, extension temperatures and switching time between them. 2) Cancer: multiple known mutations w stable nonspecific primer hybrids (kras, BRAF V 600 E) 3) Triplet repeat expansions w stable nonspecific primer hybrids (FMR-1)

Melting Curve of Primers

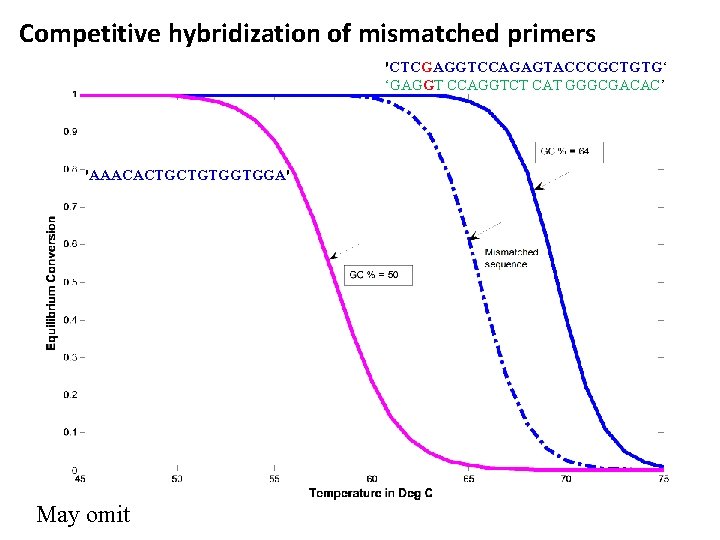
Competitive hybridization of mismatched primers 'CTCGAGGTCCAGAGTACCCGCTGTG‘ ‘GAGGT CCAGGTCT CAT GGGCGACAC’ 'AAACACTGCTGTGGTGGA' May omit

Kinetics of Multiplex Annealing

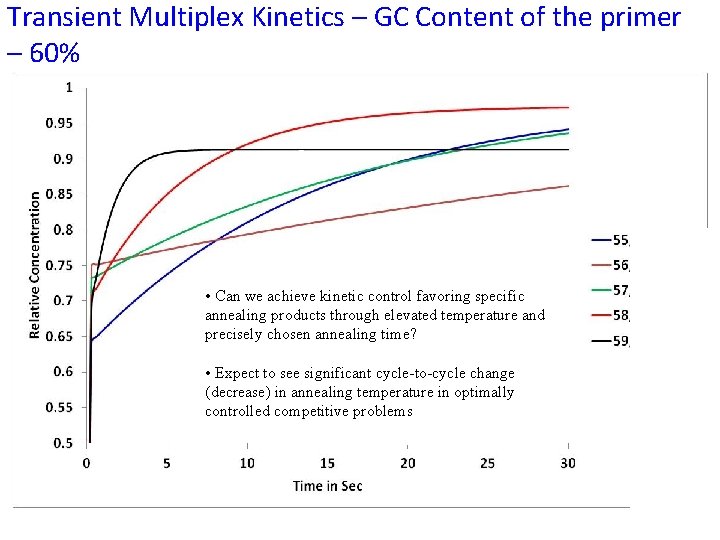
Transient Multiplex Kinetics – GC Content of the primer – 60% At lower temperature with P/S ratio approximately 1, • Can we achieve kinetic control favoring specific annealing products through elevated temperature and we could slowdown the precisely chosen annealing time? annealing reaction. • Expect to see significant cycle-to-cycle change (decrease) in annealing temperature in optimally controlled competitive problems

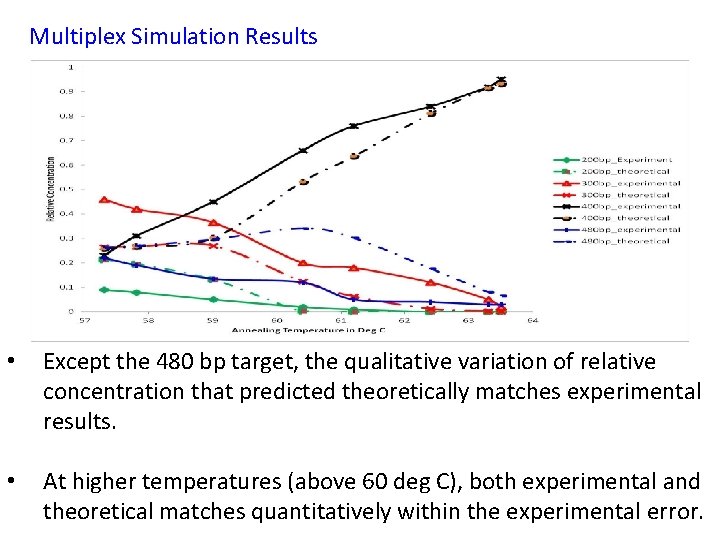
Multiplex Simulation Results • Except the 480 bp target, the qualitative variation of relative concentration that predicted theoretically matches experimental results. • At higher temperatures (above 60 deg C), both experimental and theoretical matches quantitatively within the experimental error.

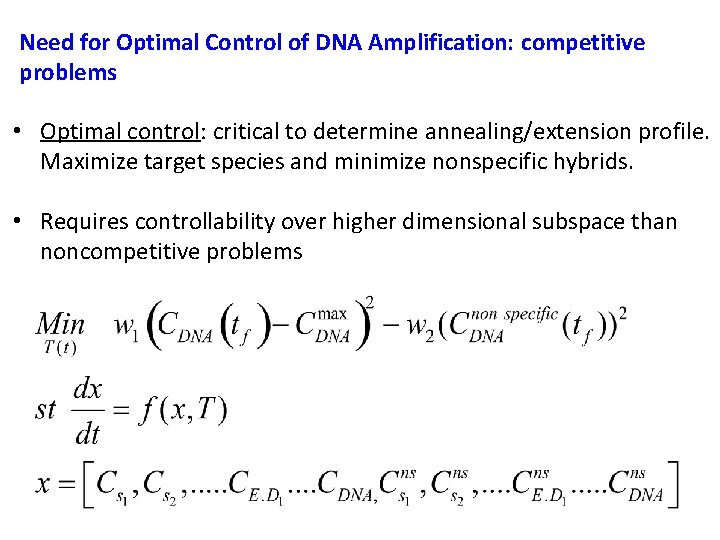
Need for Optimal Control of DNA Amplification: competitive problems • Optimal control: critical to determine annealing/extension profile. Maximize target species and minimize nonspecific hybrids. • Requires controllability over higher dimensional subspace than noncompetitive problems

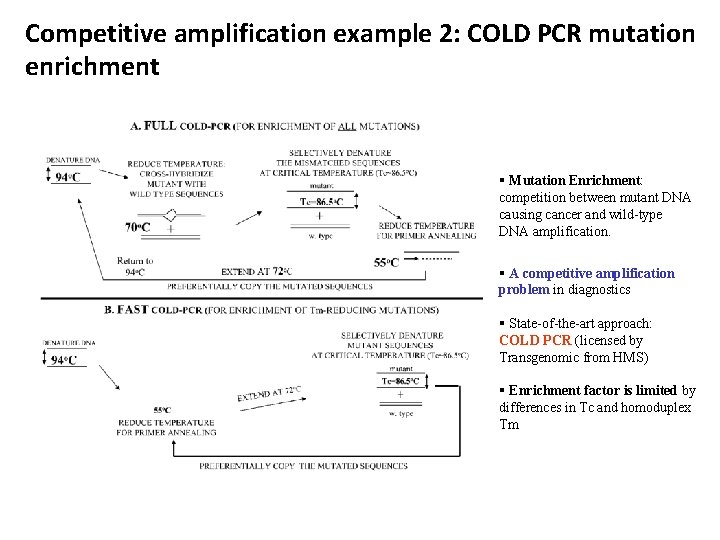
Competitive amplification example 2: COLD PCR mutation enrichment § Mutation Enrichment: competition between mutant DNA causing cancer and wild-type DNA amplification. § A competitive amplification problem in diagnostics § State-of-the-art approach: COLD PCR (licensed by Transgenomic from HMS) § Enrichment factor is limited by differences in Tc and homoduplex Tm

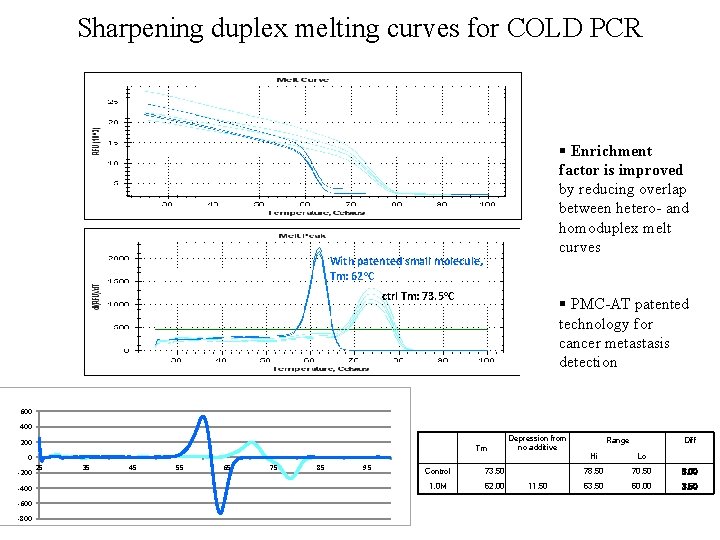
Sharpening duplex melting curves for COLD PCR § Enrichment factor is improved by reducing overlap between hetero- and homoduplex melt curves With patented small molecule, Tm: 62 o. C ctrl Tm: 73. 5 o. C § PMC-AT patented technology for cancer metastasis detection 600 400 200 0 -200 -400 -600 -800 Tm 25 35 45 55 65 75 85 95 Control 73. 50 1. 0 M 62. 00 Depression from no additive 11. 50 Range Diff Hi Lo 78. 50 70. 50 8. 00 63. 50 60. 00 3. 50

Discussion Points • NEB isothermal amplification enzymes • Next generation sequencing • Scope for interaction: – PMC-AT Software Platform to be integrated with real-time PCR software; which real-time platform? – Partnerships with thermal cycler manufacturers; NEB contacts – Use of NEB engineered polymerases

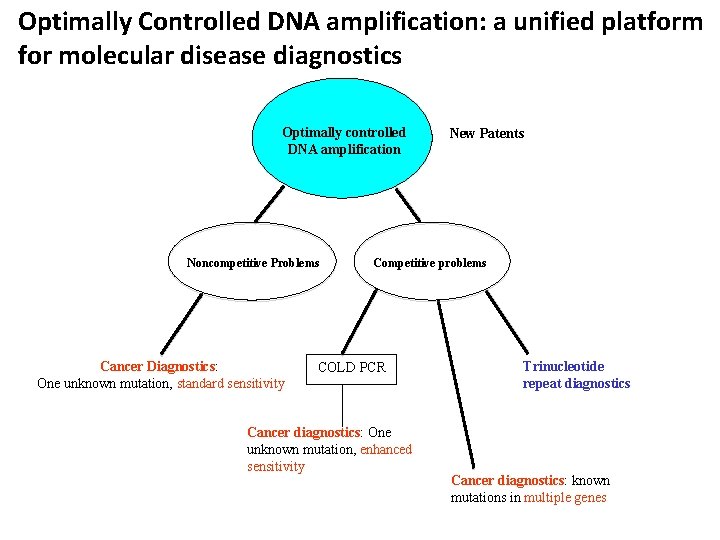
Optimally Controlled DNA amplification: a unified platform for molecular disease diagnostics Optimally controlled DNA amplification Noncompetitive Problems Cancer Diagnostics: One unknown mutation, standard sensitivity New Patents Competitive problems COLD PCR Cancer diagnostics: One unknown mutation, enhanced sensitivity Trinucleotide repeat diagnostics Cancer diagnostics: known mutations in multiple genes


Combined Annealing and Extension(Cont. ) • This shifts the equilibrium of the annealing reaction and allows the extension reaction to happen immediately. • Since Enzyme binding and extension can happen at annealing temperature, higher annealing temperature can make the extension faster even during the annealing time. In addition to this, the given extension time completes the reaction. • Whereas at lower annealing temperature, enzyme binding slow, by the time annealing time is complete, the un reacted duplexes melts at extension temperature to give back single strands. May omit

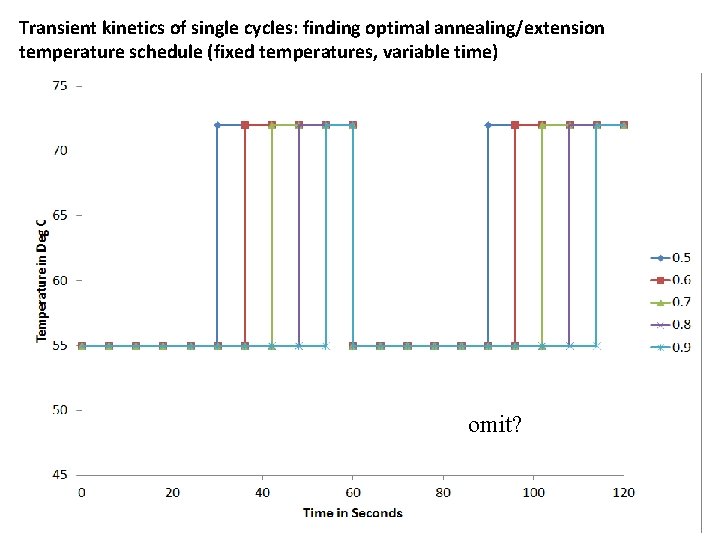
Transient kinetics of single cycles: finding optimal annealing/extension temperature schedule (fixed temperatures, variable time) omit?

Case 1 Length of the target = 800, Initial Concentration of the DNA during the start of the cycle = 2× 10 -14 M

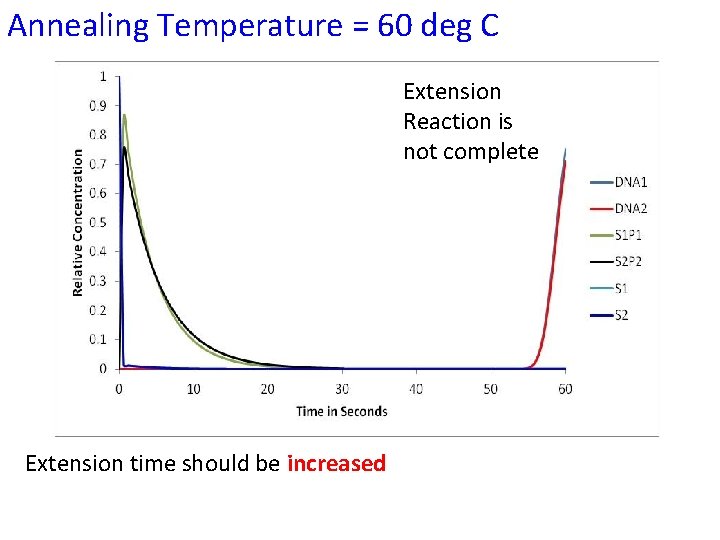
Annealing Temperature = 60 deg C Extension Reaction is not complete Extension time should be increased

Case 4 Length of the target = 800, Initial Concentration of the DNA during the start of the cycle = 2× 10 -8 M

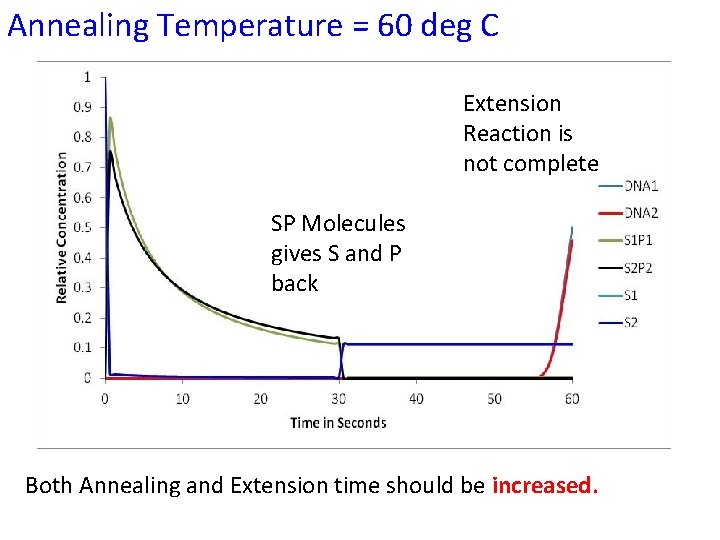
Annealing Temperature = 60 deg C Extension Reaction is not complete SP Molecules gives S and P back Both Annealing and Extension time should be increased.
