Dmitri Mendeleev 1834 1907 http en wikipedia orgwikiHenryMoseley




- Slides: 16

Dmitri Mendeleev 1834 -1907 http: //en. wikipedia. org/wiki/Henry_Moseley John Newlands 1837 -1898 http: //en. wikipedia. org/wiki/Dmitri_Mendeleev http: //www. rsc. org/education/teachers/learnnet/periodictable/scientists/newlands. htm _____ was _____ Elements & Their Properties -the ____ Periodic Table developed in the ______ by _______ late ______ 1800’s to _____ early 1900’s English _______ chemist _______, John Newlands _______ Russian _______ chemist ________, Dmitri Mendeleev and _______ English physicist _______, Henry Moseley whose ___________ arrangement of ____ elements is used _____ today Henry Moselev 1887 -1915

Elements & Their Properties I. Metals -______ metals are ______ found to the ____ left of the ____-____ stair step ____ line on the ____ Periodic _____ Table

Elements & Their Properties I. Metals -______ metals are ______ found to the ____ left of the ____-____ stair step ____ line on the ____ Periodic _____ Table Metals

Elements & Their Properties I. Metals -______ metals are _____ good _____ conductors of ____ heat and _____, electricity are ______ solids at _____ room ______ temperature (______ except ____), Mercury are _______, lustrous _____, malleable and ______ ductile Metals

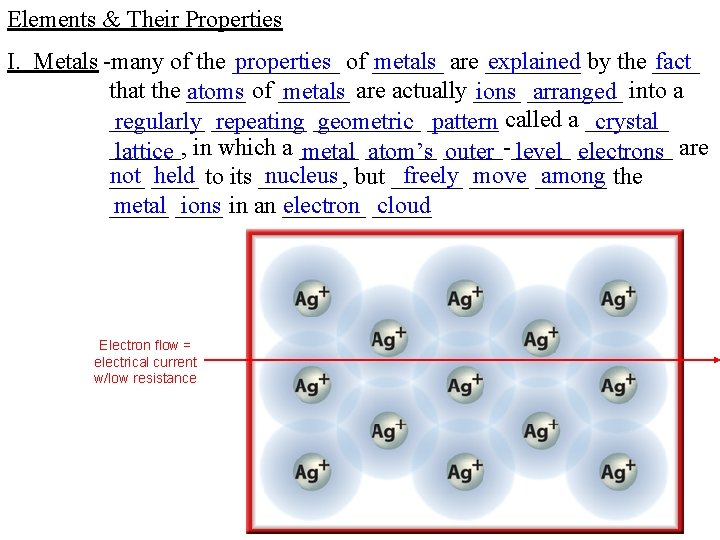
Elements & Their Properties I. Metals -many of the _____ properties of ______ metals are ____ explained by the ____ fact that the _____ atoms of ______ metals are actually ____ ions ____ arranged into a ____ regularly ____ repeating _____ geometric ______ pattern called a _______ crystal ______, lattice in which a _____ metal ______ atom’s _____-_____ outer level ____ electrons are not ____ held to its _______, nucleus but ______ freely _____ move ______ among the ___ metal ____ ions in an _______ electron _____ cloud _____ Electron flow = electrical current w/low resistance

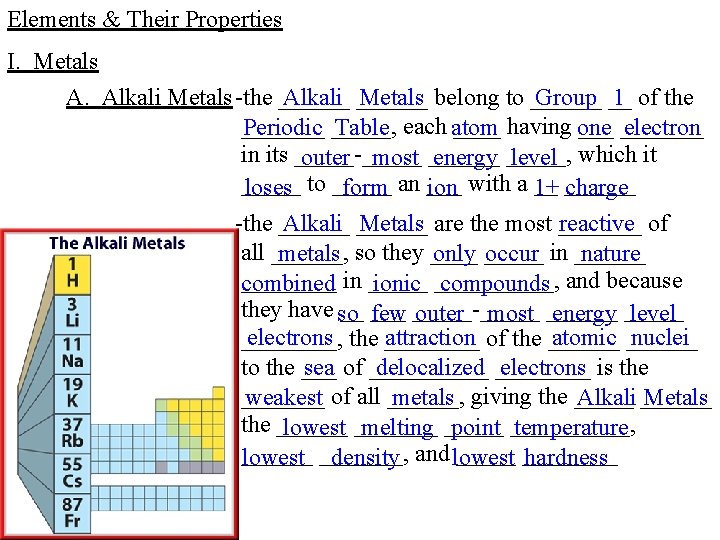
Elements & Their Properties I. Metals A. Alkali Metals -the ______ Alkali ______ Metals belong to ______ Group __ 1 of the _______ having one _______ Periodic _____, Table each atom electron in its _____-_____ outer most ______ energy _____, level which it _____ loses to _____ form an ___ ion with a __ 1+ ______ charge -the ______ Alkali ______ Metals are the most _______ reactive of all ______, metals so they ____ only _____ occur in ______ nature ____ in _____ combined ionic _____, compounds and because they have so __ ___ few _____-_____ outer most ______ energy _____ level electrons the ____ attraction of the ______ atomic ______ nuclei ____, sea of _____ delocalized ____ electrons is the to the _______ weakest of all ______, metals giving the _____ Alkali ______ Metals the ______ lowest _______ melting _____ point _____, temperature ______ lowest _______, density and lowest hardness

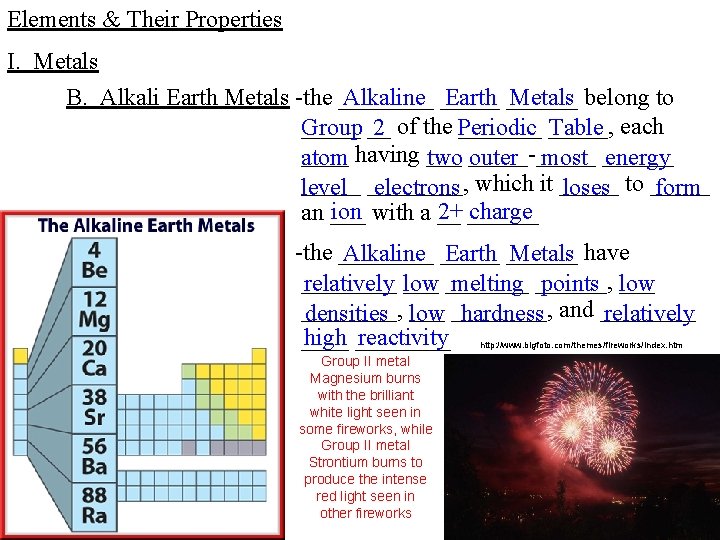
Elements & Their Properties I. Metals B. Alkali Earth Metals -the ____ Alkaline _____ Earth ______ Metals belong to _____ __ _____, Group 2 of the Periodic Table each ____ atom having ___ two _____-_____ outer most ______ energy _____ level ____, electrons which it _____ loses to _____ form ion with a __ 2+ ______ charge an ___ -the ____ Alkaline _____ Earth ______ Metals have _______ relatively low melting ______, points ___ low ____, densities ___ low ____, hardness and ____ relatively high ____ reactivity ____ http: //www. bigfoto. com/themes/fireworks/index. htm Group II metal Magnesium burns with the brilliant white light seen in some fireworks, while Group II metal Strontium burns to produce the intense red light seen in other fireworks

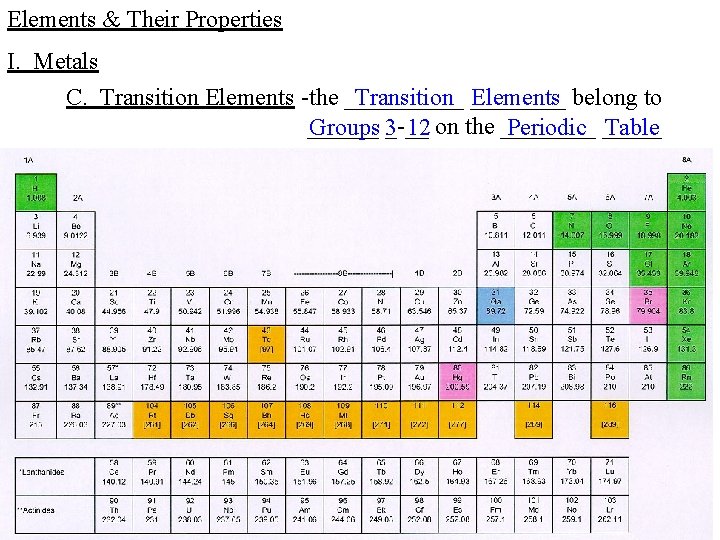
Elements & Their Properties I. Metals C. Transition Elements -the _____ Transition ____ Elements belong to ______ Groups 3_-__ 12 on the ____ Periodic _____ Table

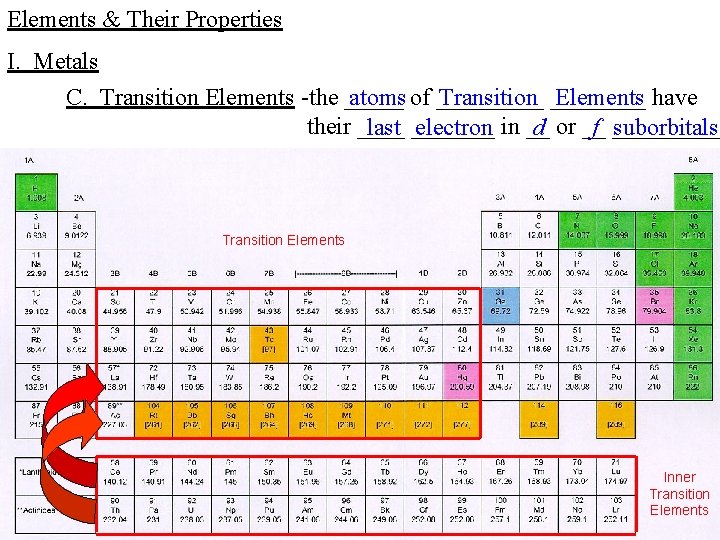
Elements & Their Properties I. Metals C. Transition Elements -the _____ atoms of _____ Transition ____ Elements have their ____ last _______ electron in __ d or __ f _____ suborbitals Transition Elements Inner Transition Elements

Elements & Their Properties http: //en. wikipedia. org/wiki/Tutankhamun http: //www. theodoregray. com/periodic. Table/Elements/074/index. html#sample 14 I. Metals C. Transition Elements -___ all _____ Transition ____ Elements have ___ two ____ electrons in their _____-____ outer most ______ energy _____, level but are also able to _____ contribute their __ d _______ orbital ____ electrons to their ___ sea delocalized _____, electrons so the of _____ attraction between their ____ ions and their ____ seas of ____ electrons is much _______, stronger giving them ______ higher ____, densities ______ higher Gold was discovered early and is highly prized for its _______ melting _____ point ______, temperatures ______ higher nonreactive properties, hardness and making them ____ less and ____, its also extremely dense and highly malleable ____ less ____ reactive http: //theodoregray. com/Periodic. Table/Elements/092/index. html#sample 1 http: //www. theodoregray. com/periodic. Table/Elements/076/index. html Tungsten is used for light bulb filaments because of its extremely high melting point temperature Osmium, the hardest metal, used in antique phonograph needles, and spent uranium used in armor-piercing projectiles because of both its hardness and its extreme density

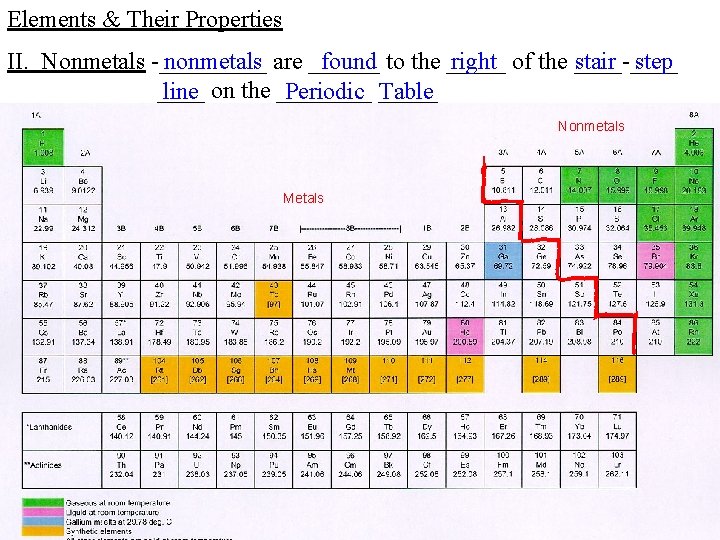
Elements & Their Properties II. Nonmetals -_____ nonmetals are ______ found to the _____ right of the ____-____ stair step ____ line on the ____ Periodic _____ Table Nonmetals Metals

Elements & Their Properties II. Nonmetals -_____ nonmetals are usually _____ gases or ______ brittle ______ solids at _____ room ______, temperature ____ poor _____ conductors of _____ electricity and ____, heat and they are ___ not ____, lustrous _____, malleable or ______ ductile -since _____ nonmetals have ____ relatively ____ high _______, electronegativity they can ______ accept ____ electrons to form _____ ionic _____, compounds share ____ electrons to form _______ covalent _____ compounds or they can _____ Diamond, the hardest known natural substance, can only by cut by other diamond. At left a 20 -carat rough diamond is cut into the 7. 6 -carat finished diamond at the right. http: //www. diamondschool. com/index. php? option=com_content&task=view&id=34&Itemid=59

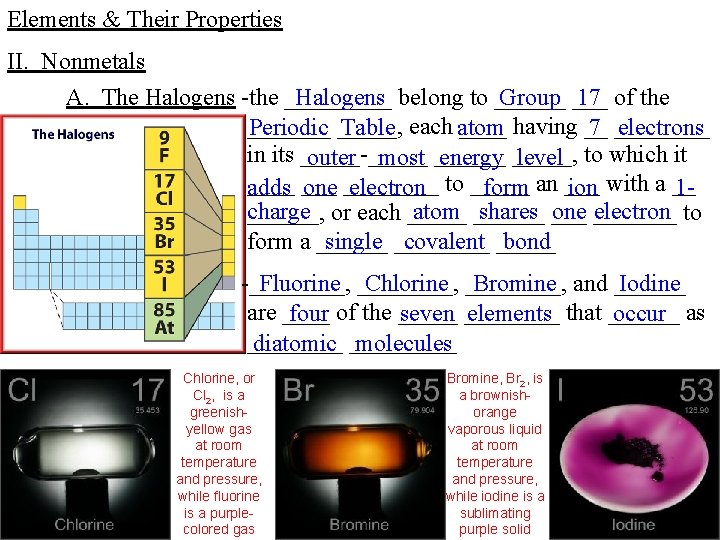
Elements & Their Properties II. Nonmetals A. The Halogens -the _____ Halogens belong to ______ Group ___ 17 of the _______ having __ Periodic _____, Table each atom 7 ____ electrons in its _____-_____ outer most ______ energy _____, level to which it ____ adds ___ one ____ electron to _____ form an ___ ion with a __ 1 charge atom ______ shares ___ one _______ electron to ______, or each _____ form a ______ single ____ covalent _____ bond Fluorine ____, Chlorine ____, Bromine and ______ Iodine -____, are ____ four of the _____ seven ____ elements that ______ occur as ____ diatomic _____ molecules Chlorine, or Cl 2, is a greenishyellow gas at room temperature and pressure, while fluorine is a purplecolored gas Bromine, Br 2, is a brownishorange vaporous liquid at room temperature and pressure, while iodine is a sublimating purple solid

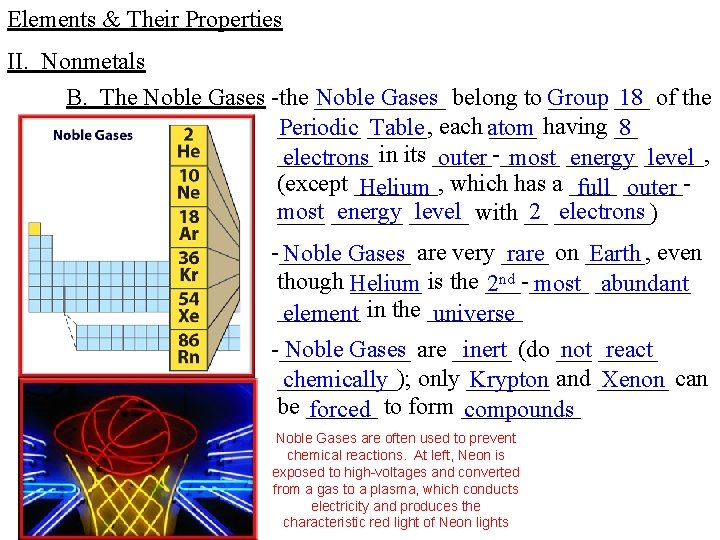
Elements & Their Properties II. Nonmetals B. The Noble Gases -the ______ Noble Gases belong to Group _____ 18 of the _______ having __ Periodic _____, Table each atom 8 ____ electrons in its _____-_____ outer most ______ energy _____, level (except _______, Helium which has a ____ full _____outer most energy _____ level with __ 2 ____) electrons ______ -______ Noble Gases are very ____ rare on _____, Earth even though Helium ______ is the ___-_____ 2 nd most ____ abundant _______ element in the ____ universe -______ Noble Gases are _____ inert (do ___ not _____ react _____); chemically only _______ Krypton and ______ Xenon can be ______ forced to form _____ compounds Noble Gases are often used to prevent chemical reactions. At left, Neon is exposed to high-voltages and converted from a gas to a plasma, which conducts electricity and produces the characteristic red light of Neon lights

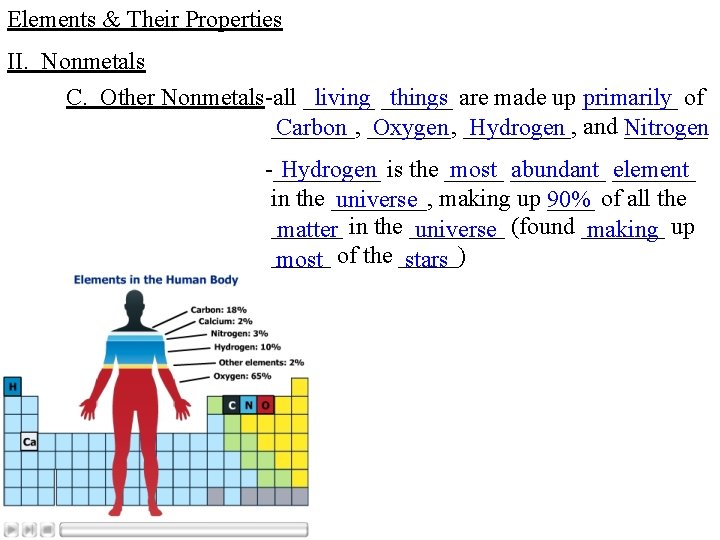
Elements & Their Properties II. Nonmetals C. Other Nonmetals-all ______ living ______ things are made up ____ primarily of _______, Carbon _______, Oxygen _____, Hydrogen and _______ Nitrogen -_____ Hydrogen is the _____ most ____ abundant _______ element in the ____, universe making up ____ 90% of all the ______ matter in the ____ universe (found _______ making up _____ most of the _____) stars

Elements & Their Properties II. Nonmetals C. Other Nonmetals-_____ Hydrogen is also very _______, reactive _______, gaining losing or _____ most _____ often _______ sharing ____ electrons ______, ionic or ____ covalent _____ compounds to form _____ Nitrogen makes up ____ 79% of the -____ atmosphere and is so _____ unreactive it is often _____, nicknamed the other _____ Noble Gas _____ Oxygen makes up ____ 21% of the -_______ atmosphere and is the most ____ abundant ____ very _____, reactive _______ element on Earth _____ The crash of the Hindenburg, (the largest aircraft ever to fly) in 1937 in Lakehurst, New Jersey demonstrates the flammability of Hydrogen and the support of combustion by Oxygen. 35 people died, yet 62 passengers and crew survived. -_____, Hydrogen ____, Nitrogen and Oxygen _______ occur in their _____ elemental _____ form as ____ diatomic ____ molecules