DMC Issues from a Pharmaceutical Industry Perspective Steven



















- Slides: 27

DMC Issues from a Pharmaceutical Industry Perspective Steven Snapinn Amgen FDA-Industry Workshop September 15, 2005

Outline • • Assessing the Need for a DMC Independence of the DMC Scope of the DMC’s Responsibilities Issues in Setting the Stopping Boundaries • How Are Decisions Made? – Case Study 1: PRISM-PLUS • Stopping for Futility – Case Study 2: CONSENSUS II

Assessing the Need for a DMC • Considerations – Seriousness of the Medical Condition – Uncertainty of Efficacy and Safety – Size of the Trial – Duration of the Trial • Benefits of Having a DMC – Credibility – Experience – Neutrality

Internal vs. External DMCs • Early Development – Safety Monitoring by Study Team • Dose Escalation Decisions – Efficacy Monitoring by Internal DMC • Independent of Study Team • Phase III – External DMC Typical

Independence of the DMC • Committee Membership – DMC Independent of Sponsor – No Sponsor Participation in Closed Session • Preparation of Interim Reports – Regulatory Guidance Frowns on This Being a Sponsor Responsibility – Sponsors Typically Contract Independent Group

The Case for an Unblinded Sponsor Statistician • “Firewall” • Familiarity with the Study • Control of the Allocation Schedule and Interim Results – Sponsor’s Risk – Quality Assurance – Data Leaks • What Constitutes “Independence”?

Scope of the DMC’s Responsibilities • Safety Monitoring • Efficacy Monitoring • Timeliness and Accuracy of the Database • Protocol Adherence? • Sample Size Re-estimation? • Requests for ad hoc Analyses

Issues in Setting the Stopping Boundaries • Stopping Boundaries for Safety • Stopping Boundaries for Efficacy: Protect Patients in the Trial or Protect All Patients? – Require Overwhelming Evidence or Moderate Evidence? – Are Patients Fully Informed?

Issues in Setting the Stopping Boundaries (continued) • Review of Efficacy Data for Risk/Benefit Assessment Only – Set Extreme Efficacy Criterion (eg, 0. 0005) – Protection for Sponsor • Group Sequential vs. Conditional Probability

Other Issues • Partial vs. Full Unblinding • Information Sharing Across DMCs • Monitoring Noninferiority Trials

How Are Decisions Made? • Decision-Making Authority – Independent Steering Committee – Sponsor’s Awkward Position • Reclaiming Type I Error – Efficacy Boundary Crossed But Trial Continues – Futility Boundaries

Case Study: PRISM-PLUS • Patients with Acute Coronary Syndrome – Non-Q-Wave Myocardial Infarction – Unstable Angina Pectoris • Evaluation of Tirofiban, an Inhibitor of Platelet Aggregation

PRISM-PLUS Study Design • Three Treatment Arms – Control Arm: Heparin Alone – Monotherapy Arm: Tirofiban Alone – Combination Arm: Heparin+Tirofiban • Composite Endpoint – Refractory Ischemia/Readmission for UAP – Myocardial Infarction – Death

Study Organization • Oversight by an Independent Steering Committee – Sponsor Representatives Attend SC Meetings – Makes Decisions on DMC Recommendations • Data Monitored by an Independent DMC – No Sponsor Representative (Other than Unblinded Statistician) – Recommendations on Trial Modification to SC

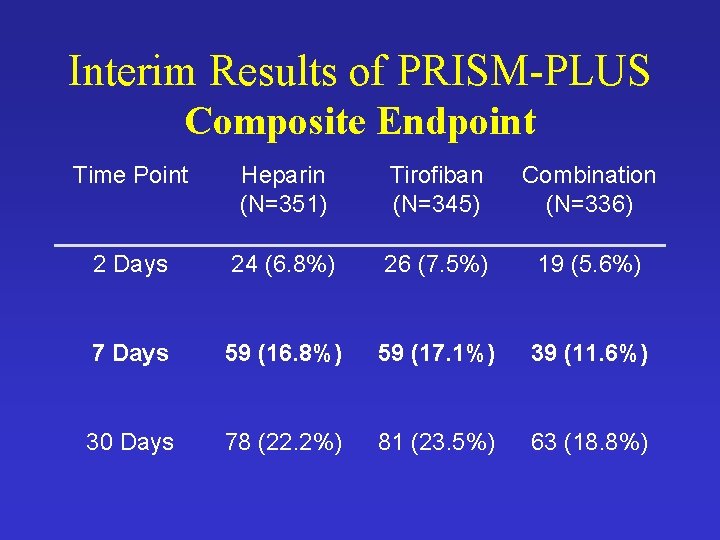
Interim Results of PRISM-PLUS Composite Endpoint Time Point Heparin (N=351) Tirofiban (N=345) Combination (N=336) 2 Days 24 (6. 8%) 26 (7. 5%) 19 (5. 6%) 7 Days 59 (16. 8%) 59 (17. 1%) 39 (11. 6%) 30 Days 78 (22. 2%) 81 (23. 5%) 63 (18. 8%)

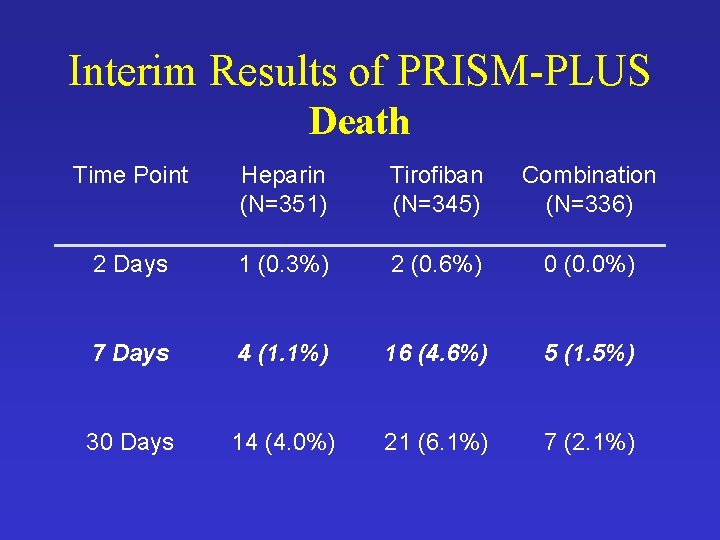
Interim Results of PRISM-PLUS Death Time Point Heparin (N=351) Tirofiban (N=345) Combination (N=336) 2 Days 1 (0. 3%) 2 (0. 6%) 0 (0. 0%) 7 Days 4 (1. 1%) 16 (4. 6%) 5 (1. 5%) 30 Days 14 (4. 0%) 21 (6. 1%) 7 (2. 1%)

Summary of Results • Composite Endpoint Rates Similar in Heparin and Tirofiban Groups • Death Rate Higher in the Tirofiban Group • 7 -Day Mortality – 16/345 vs. 4/351 - p-value = 0. 006 • Pooling Heparin and Combo Groups – 16/345 vs. 9/687 - p-value = 0. 001 • Is This Sufficient Evidence?

The Pharmaceutical Sponsor’s Dilemma • DSMB Recommended Discontinuation of the Tirofiban Arm • Steering Committee Felt That the Evidence Was Insufficient • Representatives of the Sponsor Were Present at the Meeting • Can the Sponsor Allow Randomization to Continue when the DMC Believes That Patients Will Die Unnecessarily?

Final Results of PRISM Death Time Point Heparin (N=1616) Tirofiban (N=1616) 2 Days 4 (0. 2%) 6 (0. 4%) 7 Days 25 (1. 6%) 16 (1. 0%) 30 Days 59 (3. 6%) 37 (2. 3%)

Stopping for Futility • Inability of a Trial to Meet Its Objectives – Operational vs. Statistical – Goal Is to Preserve Resources • No Ethical Imperative • Group Sequential vs. Conditional Probability – Frequentist vs. Bayesian Methods

Stopping for Futility (continued) • One-Sided vs. Two-Sided Boundaries • Can Alpha Be “Reclaimed”? • Cost in Power and Secondary Objectives • Need for Prior Agreement

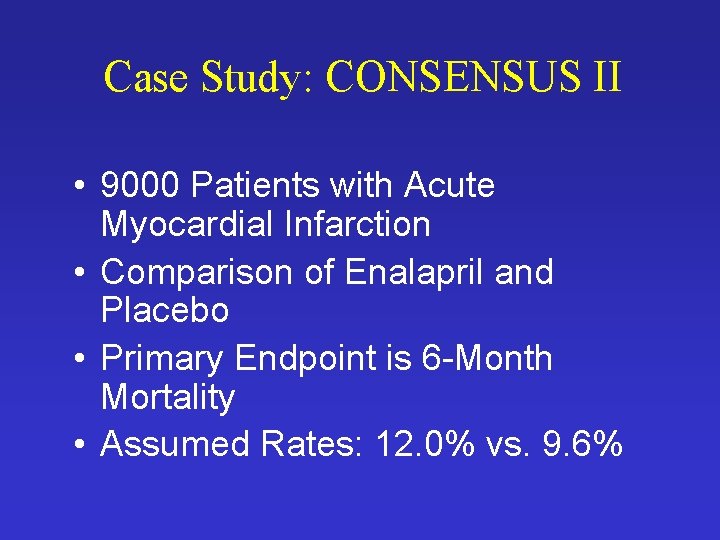
Case Study: CONSENSUS II • 9000 Patients with Acute Myocardial Infarction • Comparison of Enalapril and Placebo • Primary Endpoint is 6 -Month Mortality • Assumed Rates: 12. 0% vs. 9. 6%

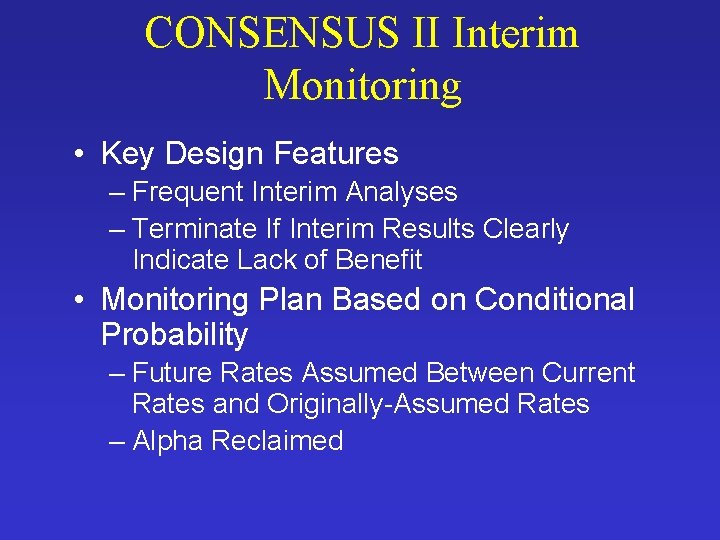
CONSENSUS II Interim Monitoring • Key Design Features – Frequent Interim Analyses – Terminate If Interim Results Clearly Indicate Lack of Benefit • Monitoring Plan Based on Conditional Probability – Future Rates Assumed Between Current Rates and Originally-Assumed Rates – Alpha Reclaimed

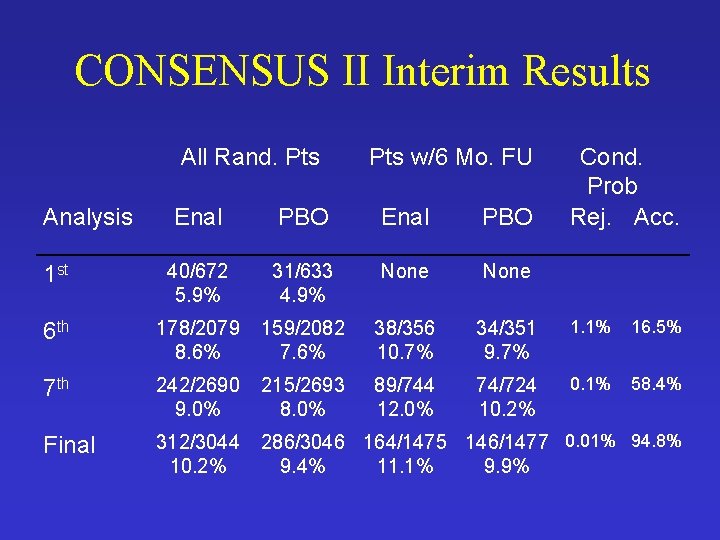
CONSENSUS II Interim Results All Rand. Pts Analysis Pts w/6 Mo. FU Cond. Prob Rej. Acc. Enal PBO 1 st 40/672 5. 9% 31/633 4. 9% None 6 th 178/2079 8. 6% 159/2082 7. 6% 38/356 10. 7% 34/351 9. 7% 1. 1% 16. 5% 7 th 242/2690 9. 0% 215/2693 8. 0% 89/744 12. 0% 74/724 10. 2% 0. 1% 58. 4% Final 312/3044 10. 2% 286/3046 164/1475 146/1477 0. 01% 94. 8% 9. 4% 11. 1% 9. 9%


CONSENSUS II Lessons Learned • Stopping for futility is a difficult problem – Without Clear Trend Toward Efficacy or Harm There’s No Ethical Imperative – Without Hope for Benefit Patients Should Not Be Subjected to Risks – Prior Agreement Required • Within DMC • Between DMC and Trial Leadership

CONSENSUS II Lessons Learned (continued) • Stopping Boundary – Flexibility Is an Advantage – Reclaiming Alpha May Not Be Appropriate – Basing Conditional Probabilities Only on Patients With Complete Follow-Up Was a Disadvantage