Divisions of Matter Pure Substances and Mixtures Pure

+ Divisions of Matter Pure Substances and Mixtures


+ Pure Substance n Cannot n Has be broken down and retain its properties. a fixed composition. n Every sample of a pure substance has EXACTLY the same composition AND exactly the same characteristics - homogeneous n Examples n Salt Na. Cl n Copper n Water – H 2 O is always 11. 2% Hydrogen and 88. 8% Oxygen
+ Elements n Simplest form of matter n Made up of ONE type of ATOM n All atoms in an element have same number of protons in nucleus. n Atom- Smallest unit of an elements that KEEPS the IDENTITY of the element
+ PERIODIC TABLE n Elements are represented by symbols n NEED to know Symbols. (handout) n 92 naturally occurring elements n 93 -112 Made only under lab conditions n Information on periodic table n Symbol n Atomic # - which tells us the number of protons n Atomic Mass – Average mass of the element (Number of neutrons plus protons)
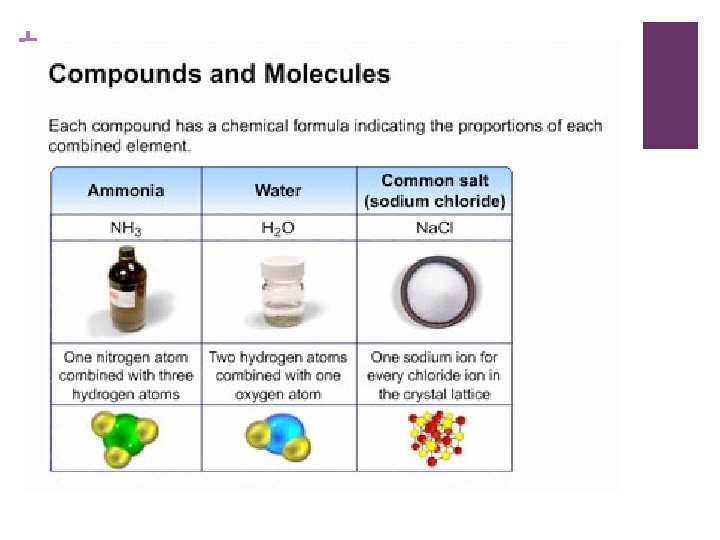
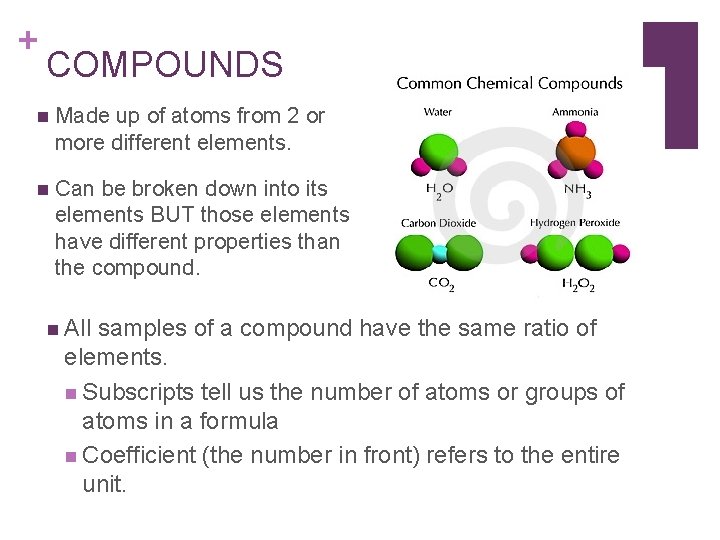
+ COMPOUNDS n Made up of atoms from 2 or more different elements. n Can be broken down into its elements BUT those elements have different properties than the compound. n All samples of a compound have the same ratio of elements. n Subscripts tell us the number of atoms or groups of atoms in a formula n Coefficient (the number in front) refers to the entire unit.

+ Law of conservation of Energy The total amount of energy remains the same in a system. Energy can be absorbed or released in a system, it can change forms, but it is not destroyed or created. PAGE 11 IN YOUR BOOK

+ MIXTURES A mixture is a blend of two or more kinds of matter, each of which retains it’s own identity and properties.

Web site to interact with KS 3 Bitesize http: //www. bbc. co. uk/schools/ks 3 bitesize/science

+ Mixtures http: //www. bbc. co. uk/schools/ks 3 bitesize/science n Homogeneous n Uniform composition n Same proportions of components throughout n Also called solutions n Examples n Salt water n Sugar water n Stainless steel n Heterogeneous n NOT uniform throughout n n Heavier particles may concentrate at bottom of container 2 types/ colloids & suspensions n Examples n Granite n Wood n Blood n Clay in water
+ Heterogeneous mixtures Suspensions n Often large visible particles that tend to settle out n Tend to block light n Example: corn starch and water, clay and water Colloids n Often suspended microscopic particles n Exhibit Tyndale effect – which is when light is scattered by very small particles in its path , it makes a beam of light visible n Example: gelatin milk, mayonnaise in water,
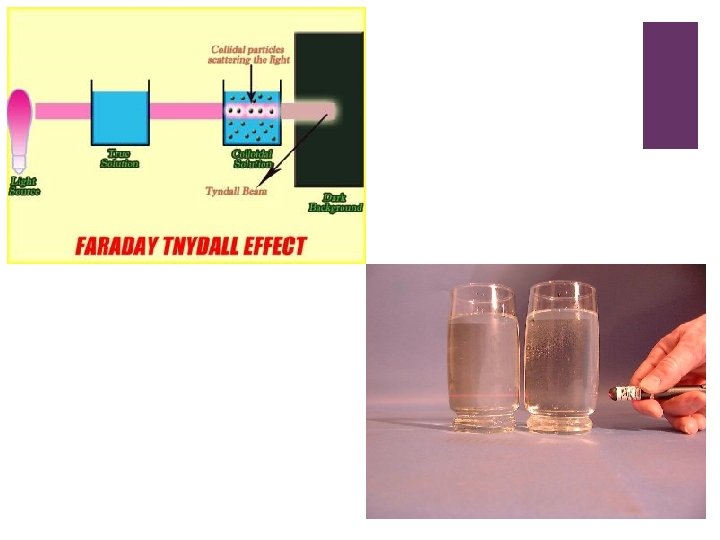
+ Tyndale effect:
- Slides: 13