Disturbing Chemical Equilibrium Relationship Between Kc and Kp

Disturbing Chemical Equilibrium

Relationship Between Kc and Kp • Plugging this into the expression for Kp for each substance, the relationship between Kc and Kp becomes where Kp = Kc (RT) n n = (moles of gaseous product) - (moles of gaseous reactant)

Relationship Between Kc and Kp 2 Cl 2(g) + 2 H 2 O(g) 4 HCl(g) + O 2(g) Kp = 0. 0752 at 480°C Kp = PHCl 4·PO 2 PCl 22·PH 2 O 2 Kc = ? Kp = Kc(RT)n 0. 0752 = Kc(0. 0821∙ 753)1 0. 0752 = Kc(61. 8213) Kc = 1. 22 x 10 -3

Disturbing a Chemical Equilibrium

Disturbing a Chemical Equilibrium • Equilibrium may be disturbed 3 ways: 1. By changing the concentration of a reactant or product 2. By changing the volume (for systems of gases) 3. By changing the temperature

Disturbing a Chemical Equilibrium 1. By changing the concentration of a reactant or product – If the concentration of a reactant or product is changed from its equilibrium value at a given temperature, equilibrium will be reestablished eventually • New equilibrium concentrations will be different, but the equilibrium constant will be the same

Disturbing a Chemical Equilibrium • Assume equilibrium has been established in a 1. 00 -L flask with [butane] = 0. 500 mol/L and [isobutane] = 1. 25 mol/L Butane Isobutane K = 2. 5 Then 1. 50 mol of butane is added. What are the concentrations of butane and isobutane when equilibrium is reestablished?
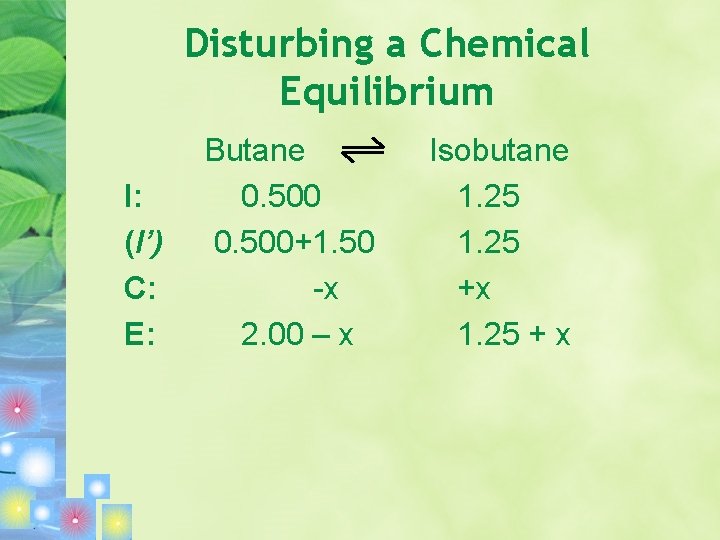
Disturbing a Chemical Equilibrium I: (I’) C: E: Butane 0. 500+1. 50 -x 2. 00 – x Isobutane 1. 25 +x 1. 25 + x
![Disturbing a Chemical Equilibrium K = [isobutane]/[butane] = 2. 5 (1. 25 + x)/ Disturbing a Chemical Equilibrium K = [isobutane]/[butane] = 2. 5 (1. 25 + x)/](http://slidetodoc.com/presentation_image_h/2d0d7c66d20f3f95ce0480ee46db8082/image-9.jpg)
Disturbing a Chemical Equilibrium K = [isobutane]/[butane] = 2. 5 (1. 25 + x)/ (2. 00 -x) = 2. 5(2. 00 –x) = 1. 25 + x 5 – 2. 5 x = 1. 25 + x 3. 75 = 3. 5 x X = 1. 07 mol/L [butane] = (0. 500 +1. 50 – x) = 0. 93 M [isobutane] = 1. 25 + 1. 07 = 2. 32 M

Disturbing a Chemical Equilibrium 2. By changing the volume (for systems of gases • For rxns involving gases, the stress of a volume decrease (increase in pressure) will be counterbalanced by a change in the equilibrium composition to one having a smaller number of gas molecules • For a volume increase (decrease in pressure), the equilibrium will favor the side of the rxn with the larger number of gas molecules • For a rxn in which there is no change in the number of gas molecules, a volume change will have no effect

Disturbing a Chemical Equilibrium • The formation of ammonia from its elements is an important industrial process: 3 H 2(g) + N 2(g) 2 NH 3(g) – How does the equilibrium composition change when extra H 2 is added? • Shifts it to the right – When extra NH 3 is added? • Shifts it to the left – What is the effect on the equilibrium when the volume of the system is increased? • Shifts it the left

Disturbing a Chemical Equilibrium 3. By changing the temperature • • Difficult because equilibrium changes with temperature If you know whether an experiment is endothermic or exothermic you can make a qualitative prediction – – – When the temperature of a system at equilibrium increases, the equilibrium will shift in the direction that absorbs energy as heat If the temperature decreases, the equilibrium will shift in the direction that releases energy as heat – that is, in the exothermic direction. Changing the temperature changes the value of K

Disturbing a Chemical Equilibrium 2 NOCl(g) 2 NO(g) +Cl 2(g) Δr. H°= +77. 1 k. J Does the equilibrium concentration of NOCl increase or decrease as the temperature of the system is increased? – It decreases
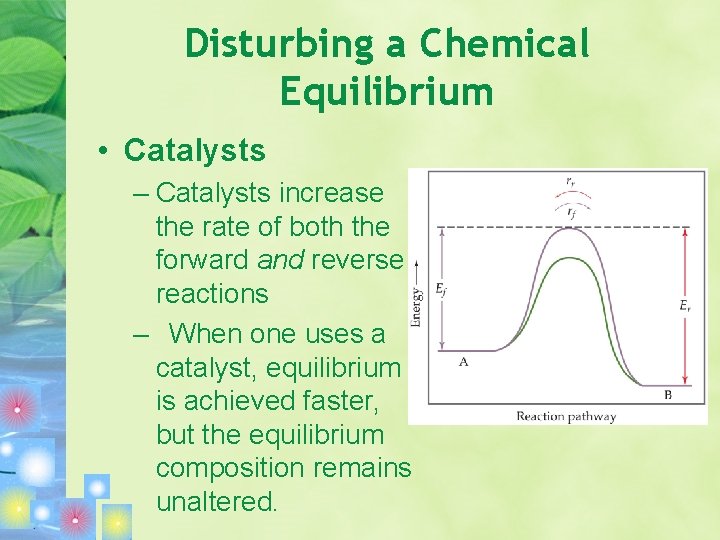
Disturbing a Chemical Equilibrium • Catalysts – Catalysts increase the rate of both the forward and reverse reactions – When one uses a catalyst, equilibrium is achieved faster, but the equilibrium composition remains unaltered.
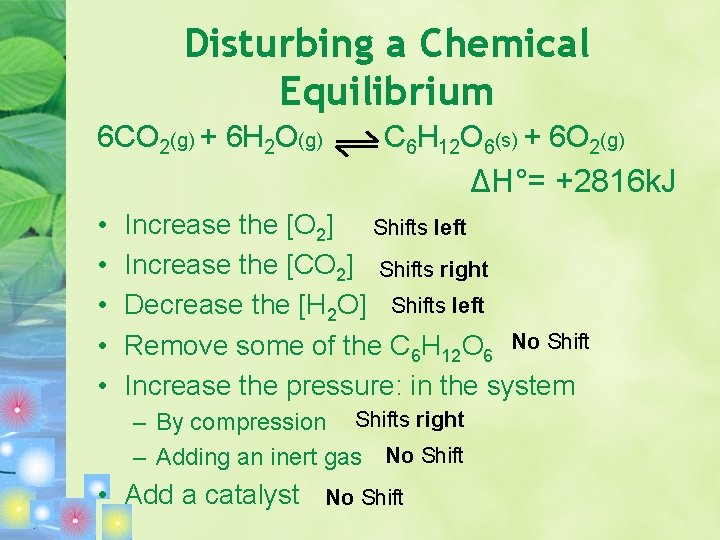
Disturbing a Chemical Equilibrium 6 CO 2(g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) ΔH°= +2816 k. J • • • Increase the [O 2] Shifts left Increase the [CO 2] Shifts right Decrease the [H 2 O] Shifts left Remove some of the C 6 H 12 O 6 No Shift Increase the pressure: in the system – By compression Shifts right – Adding an inert gas No Shift • Add a catalyst No Shift
- Slides: 15