DISTINGUISHING TEST BETWEEN ORGANIC COMPOUNDS METHANOL AND ETHANOL

DISTINGUISHING TEST BETWEEN ORGANIC COMPOUNDS METHANOL AND ETHANOL GIVES IODOFORM TEST. WHERE AS METHANOL DOES NOT CH 3 CH 2 OH +NAOI NAOH, I 2 CH 3 CHO+NAI+H 2 O CH 3 CHO+3 NAOI- CHI 3+HCOONA+2 NAOH CH 3 OH+-NAOI---. >NO REACTION

Etanol and Phenol Ethanol gives iodofrm test, where as phenol dose not CH 3 CH 2 OH+Na. OI CHI 3+HCOONa+Na. OH C 6 H 5 OH+Na. OI NO reaction Phenol gives violet colour with Fe. Cl 3 3 C 6 H 5 OH+Fe. Cl 3 (C 6 H 5 O)3 Fe+3 HCl
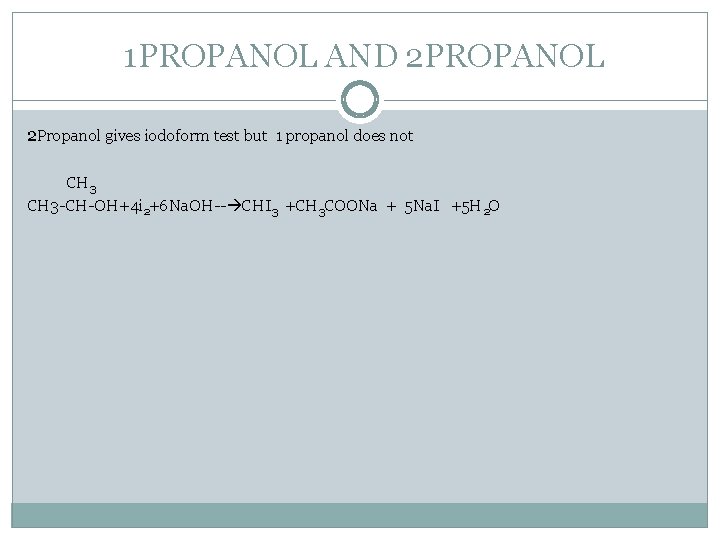
1 PROPANOL AND 2 PROPANOL 2 Propanol gives iodoform test but 1 propanol does not CH 3 -CH-OH+4 i 2+6 Na. OH-- CHI 3 +CH 3 COONa + 5 Na. I +5 H 2 O
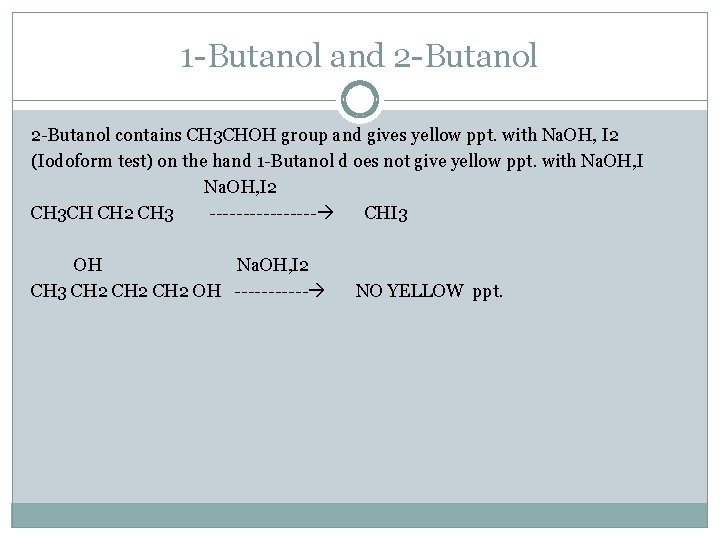
1 -Butanol and 2 -Butanol contains CH 3 CHOH group and gives yellow ppt. with Na. OH, I 2 (Iodoform test) on the hand 1 -Butanol d oes not give yellow ppt. with Na. OH, I 2 CH 3 CH CH 2 CH 3 -------- CHI 3 OH Na. OH, I 2 CH 3 CH 2 OH ------ NO YELLOW ppt.
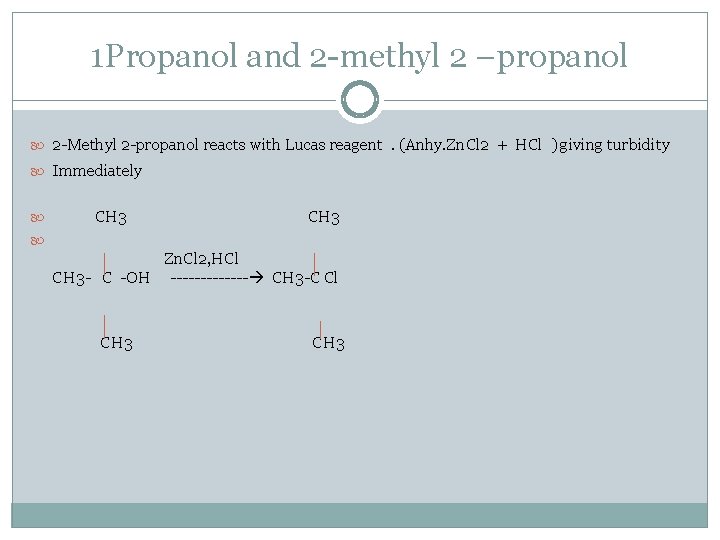
1 Propanol and 2 -methyl 2 –propanol 2 -Methyl 2 -propanol reacts with Lucas reagent. (Anhy. Zn. Cl 2 + HCl ) giving turbidity Immediately CH 3 Zn. Cl 2, HCl CH 3 - C -OH ------- CH 3 -C Cl CH 3
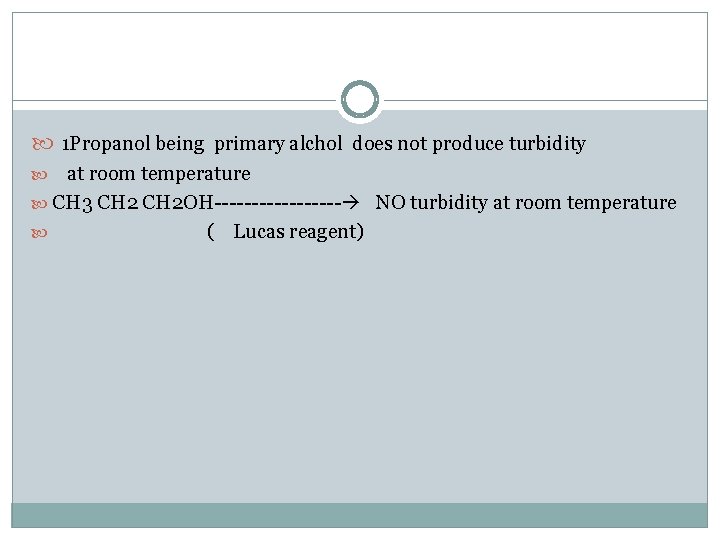
1 Propanol being primary alchol does not produce turbidity at room temperature CH 3 CH 2 OH--------- NO turbidity at room temperature ( Lucas reagent)
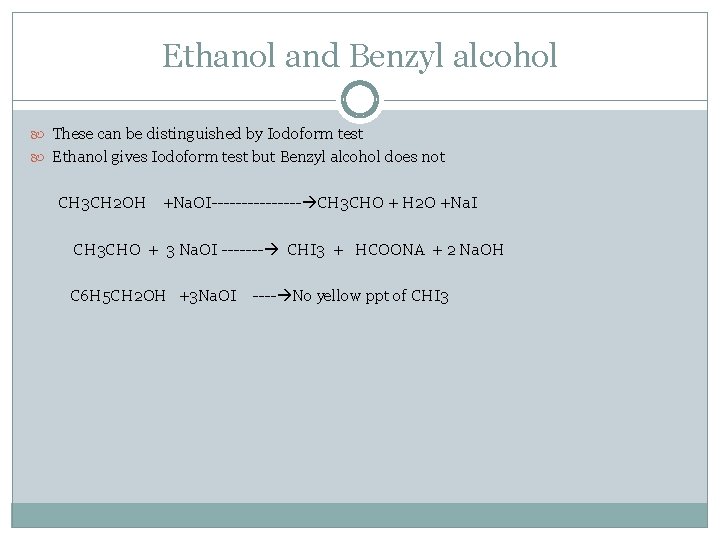
Ethanol and Benzyl alcohol These can be distinguished by Iodoform test Ethanol gives Iodoform test but Benzyl alcohol does not CH 3 CH 2 OH +Na. OI-------- CH 3 CHO + H 2 O +Na. I CH 3 CHO + 3 Na. OI ------- CHI 3 + HCOONA + 2 Na. OH C 6 H 5 CH 2 OH +3 Na. OI ---- No yellow ppt of CHI 3
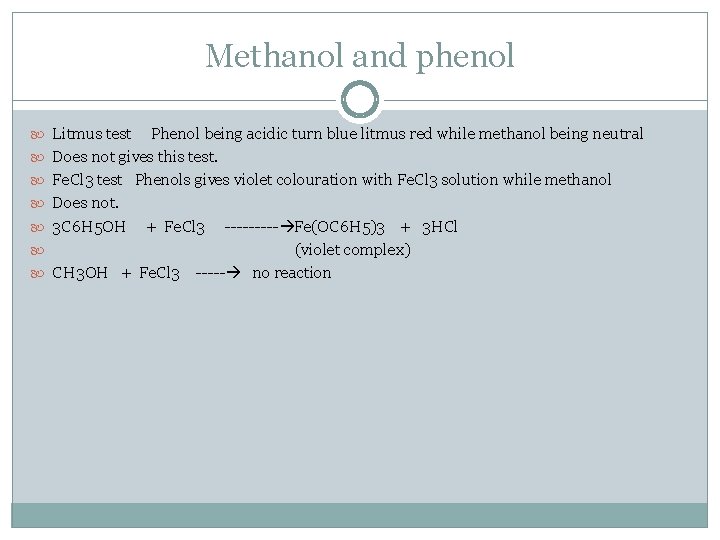
Methanol and phenol Litmus test Phenol being acidic turn blue litmus red while methanol being neutral Does not gives this test. Fe. Cl 3 test Phenols gives violet colouration with Fe. Cl 3 solution while methanol Does not. 3 C 6 H 5 OH + Fe. Cl 3 ----- Fe(OC 6 H 5)3 + 3 HCl (violet complex) CH 3 OH + Fe. Cl 3 ----- no reaction

Formaldeyhyde and Acetyaldehyde Formaldehyde gives yellow ppt with an alkaline solution ofiodine CH 3 CHO +4 Na. OH +3 I 2 ------ CHI 3 Formaldeyhyde does not give this test. + HCOONa +3 H 2 O +3 Nai
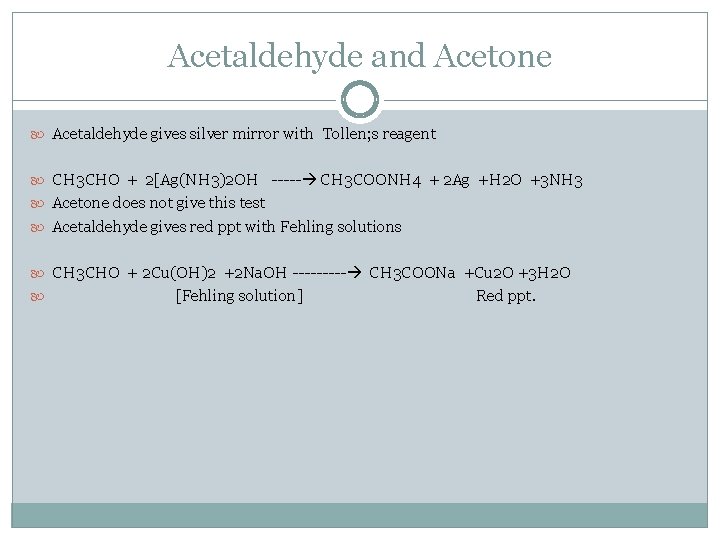
Acetaldehyde and Acetone Acetaldehyde gives silver mirror with Tollen; s reagent CH 3 CHO + 2[Ag(NH 3)2 OH ----- CH 3 COONH 4 + 2 Ag +H 2 O +3 NH 3 Acetone does not give this test Acetaldehyde gives red ppt with Fehling solutions CH 3 CHO + 2 Cu(OH)2 +2 Na. OH ----- CH 3 COONa +Cu 2 O +3 H 2 O [Fehling solution] Red ppt.
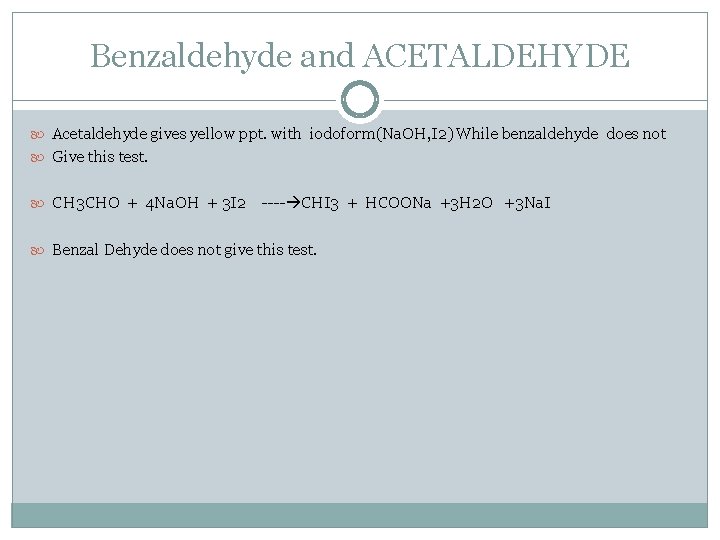
Benzaldehyde and ACETALDEHYDE Acetaldehyde gives yellow ppt. with iodoform(Na. OH, I 2) While benzaldehyde does not Give this test. CH 3 CHO + 4 Na. OH + 3 I 2 ---- CHI 3 + HCOONa +3 H 2 O +3 Na. I Benzal Dehyde does not give this test.
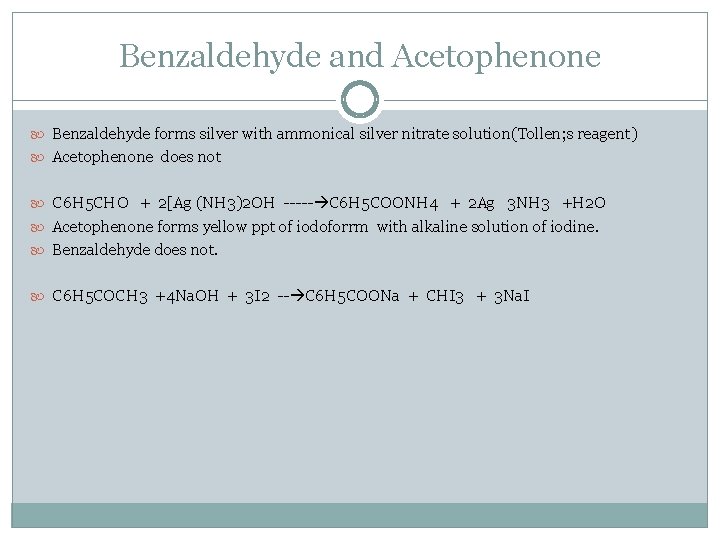
Benzaldehyde and Acetophenone Benzaldehyde forms silver with ammonical silver nitrate solution(Tollen; s reagent) Acetophenone does not C 6 H 5 CHO + 2[Ag (NH 3)2 OH ----- C 6 H 5 COONH 4 + 2 Ag 3 NH 3 +H 2 O Acetophenone forms yellow ppt of iodoforrm with alkaline solution of iodine. Benzaldehyde does not. C 6 H 5 COCH 3 +4 Na. OH + 3 I 2 -- C 6 H 5 COONa + CHI 3 + 3 Na. I
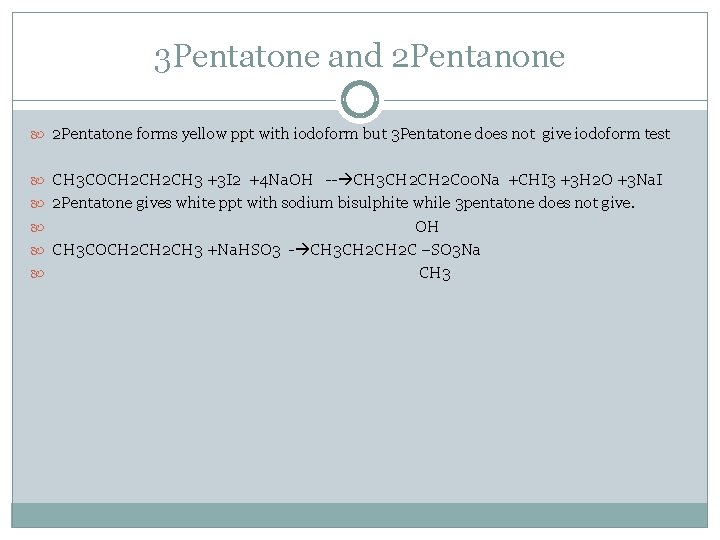
3 Pentatone and 2 Pentanone 2 Pentatone forms yellow ppt with iodoform but 3 Pentatone does not give iodoform test CH 3 COCH 2 CH 3 +3 I 2 +4 Na. OH -- CH 3 CH 2 C 00 Na +CHI 3 +3 H 2 O +3 Na. I 2 Pentatone gives white ppt with sodium bisulphite while 3 pentatone does not give. OH CH 3 COCH 2 CH 3 +Na. HSO 3 - CH 3 CH 2 C –SO 3 Na CH 3

DIFFERENCE BETWEEN FORMIC ACID AND ACETIC ACID Formic acid give Tollen; s reagent test. HCOOH + 2 [Ag(NH 3)2]+ + 2 OH- → 2 Ag + CO 2 + 2 H 2 O + 4 NH 3 Acetic acid does not give this test
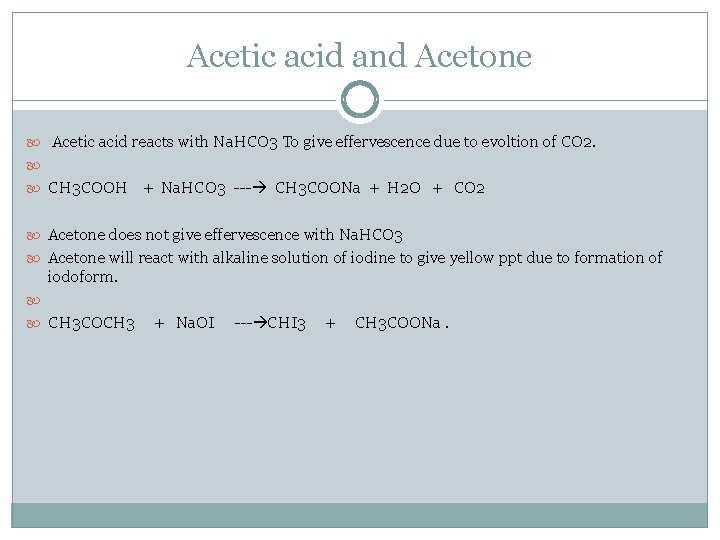
Acetic acid and Acetone Acetic acid reacts with Na. HCO 3 To give effervescence due to evoltion of CO 2. CH 3 COOH + Na. HCO 3 --- CH 3 COONa + H 2 O + CO 2 Acetone does not give effervescence with Na. HCO 3 Acetone will react with alkaline solution of iodine to give yellow ppt due to formation of iodoform. CH 3 COCH 3 + Na. OI --- CHI 3 + CH 3 COONa.
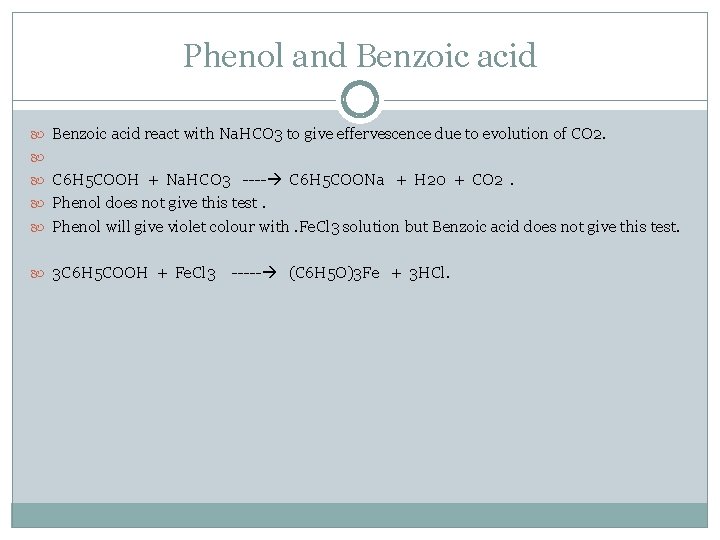
Phenol and Benzoic acid react with Na. HCO 3 to give effervescence due to evolution of CO 2. C 6 H 5 COOH + Na. HCO 3 ---- C 6 H 5 COONa + H 20 + CO 2. Phenol does not give this test. Phenol will give violet colour with. Fe. Cl 3 solution but Benzoic acid does not give this test. 3 C 6 H 5 COOH + Fe. Cl 3 ----- (C 6 H 5 O)3 Fe + 3 HCl.
Phenol and Acetic acid . Acetic react with Na. HCO 3 to give effervescence due to evolution of CO 2 CH 3 COOH + Na. HCO 3 ------ CH 3 COONa + CO 2 + H 2 O Phenol does not give this test C 6 H 5 COOH + Na. HCO 3 ---- No reaction Phenol gives violet colour with Fe. Cl 3 but acetic acid gives buff coloured ppt. 3 C 6 H 5 OH + Fe. Cl 3 -- (C 6 H 5 O)3 + 3 HCl 3 CH 3 COOH + Fe. Cl 3 ---. . (CH 3 COO)3 Fe + 3 HCl Buff ppt
Ethanol and Acetic acid Aceetic acid give effervescence with Na. HCO 3 due to liberation of CO 2 CH 3 COOH + Na. HCO 3 ---- CH 3 COONa + H 2 O +CO 2 Ethanol gives yellow ppt eith alkaline solution of I 2 , butacetic does not give this test. Na. OH , I 2 CH 3 COOH ------------ CHI 3 + HCOONa
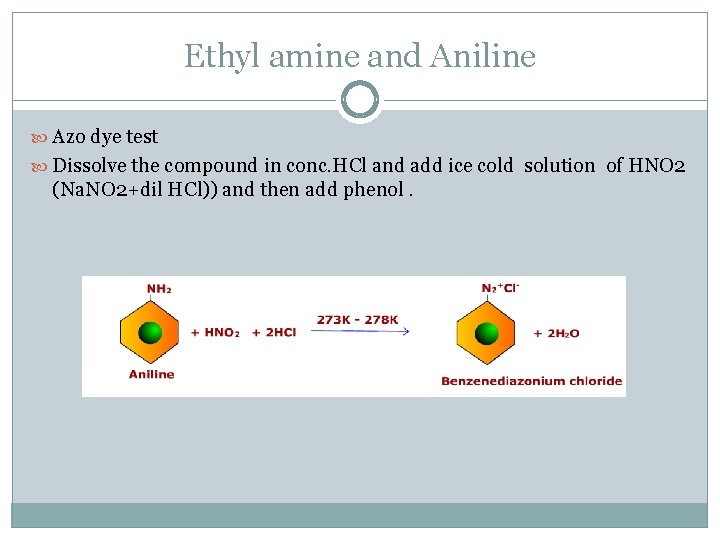
Ethyl amine and Aniline Azo dye test Dissolve the compound in conc. HCl and add ice cold solution of HNO 2 (Na. NO 2+dil HCl)) and then add phenol.
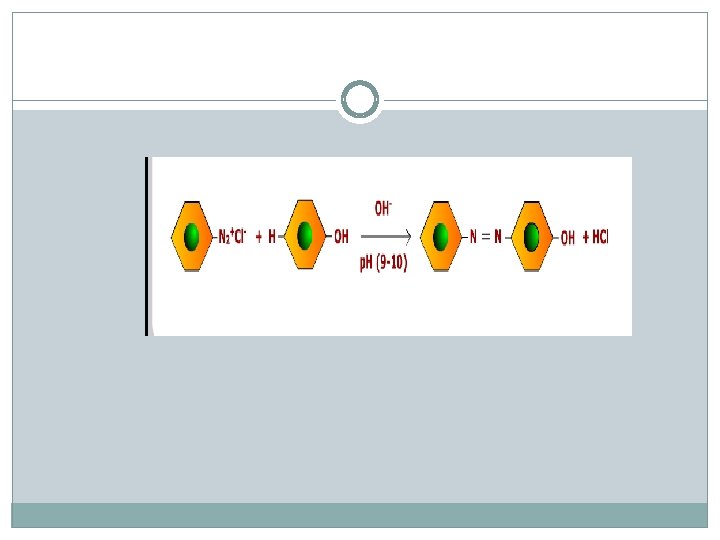

Ethyl amine does not give this test.
- Slides: 21