DISTINGUISHING BETWEEN ATOMS Chapter 4 Section 4 Identifying

DISTINGUISHING BETWEEN ATOMS Chapter 4 Section 4

Identifying Atoms � � � There about 118 known types of atoms. Each element has it’s own type of atom. All atoms of an element have to have one common factor, just like all cells of a particular person will have the same DNA. The identifying factor of an atom is the ATOMIC NUMBER. The atomic number is the number of p+ in the atom.
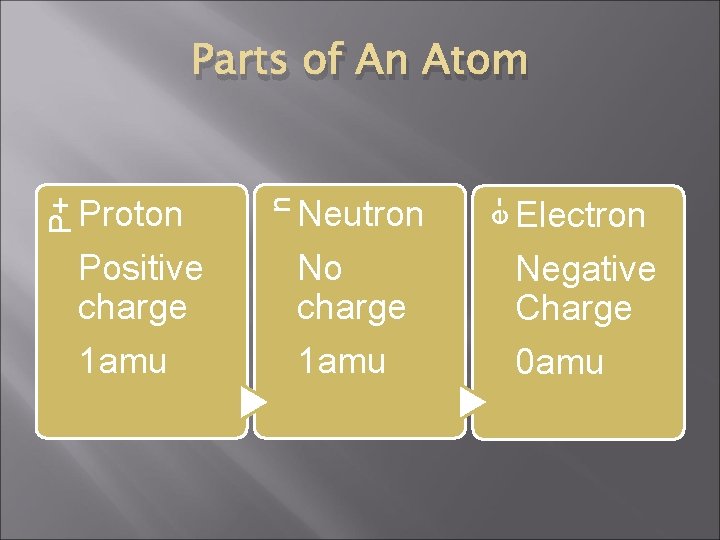
Neutron e- Proton n P+ Parts of An Atom Electron Positive charge No charge Negative Charge 1 amu 0 amu

The Nucleus � � � contains protons and neutrons The number of protons in all atoms of the same element will be the same is positively charged Contains all of the mass of the atom Each proton and each neutron will add 1 amu to the mass of the atom The atomic number (found on the periodic table) is the number of protons

Atomic Mass � The atomic mass of an atom is equal to the number of protons + the number of neutrons Atomic mass = p+ + n � � To find the number of neutrons, subtract the atomic number from the atomic mass Ex: Phosphorus atomic number = 15 15 p+ 16 n mass # = 31 number of neutron = 31 -15 16 neutrons
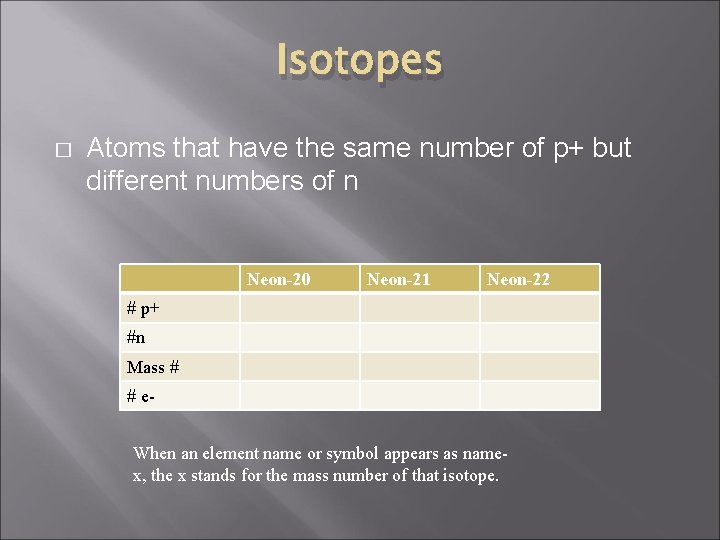
Isotopes � Atoms that have the same number of p+ but different numbers of n Neon-20 Neon-21 Neon-22 # p+ #n Mass # # e. When an element name or symbol appears as namex, the x stands for the mass number of that isotope.
Average Atomic Mass � � � The number appearing on the periodic table is actually an average atomic mass This number is the weighted average of the masses of the isotopes of an element This number accounts for the relative abundance of different isotopes of the element
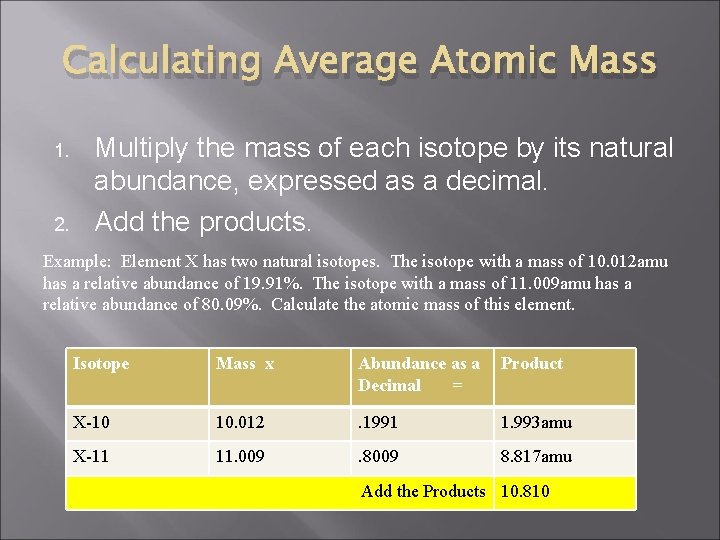
Calculating Average Atomic Mass 1. 2. Multiply the mass of each isotope by its natural abundance, expressed as a decimal. Add the products. Example: Element X has two natural isotopes. The isotope with a mass of 10. 012 amu has a relative abundance of 19. 91%. The isotope with a mass of 11. 009 amu has a relative abundance of 80. 09%. Calculate the atomic mass of this element. Isotope Mass x Abundance as a Decimal = Product X-10 10. 012 . 1991 1. 993 amu X-11 11. 009 . 8009 8. 817 amu Add the Products 10. 810
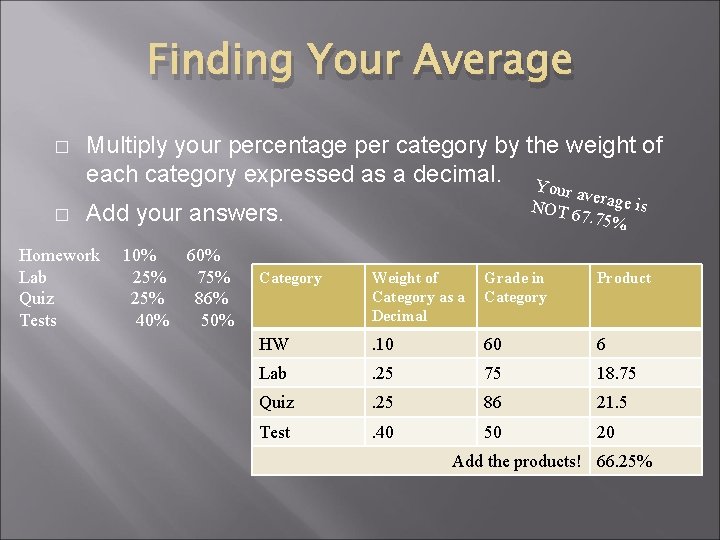
Finding Your Average � � Multiply your percentage per category by the weight of each category expressed as a decimal. Yo ur aver NOT 6 age is 7. 75% Add your answers. Homework Lab Quiz Tests 10% 60% 25% 75% 25% 86% 40% 50% Category Weight of Category as a Decimal Grade in Category Product HW . 10 60 6 Lab . 25 75 18. 75 Quiz . 25 86 21. 5 Test . 40 50 20 Add the products! 66. 25%
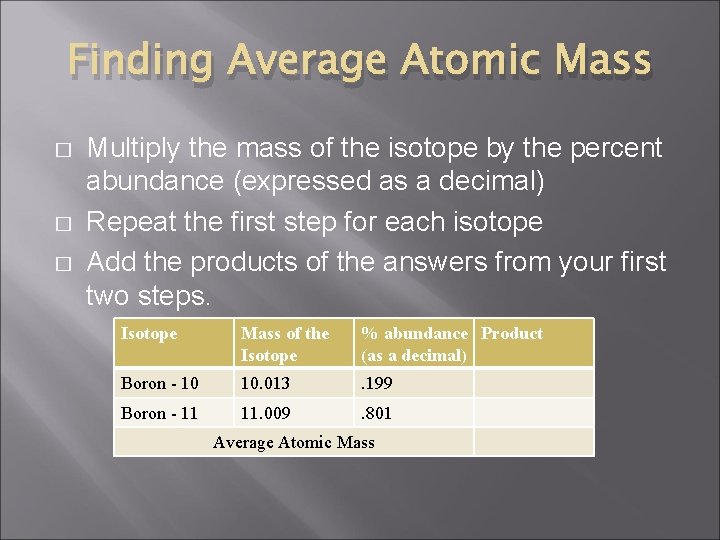
Finding Average Atomic Mass � � � Multiply the mass of the isotope by the percent abundance (expressed as a decimal) Repeat the first step for each isotope Add the products of the answers from your first two steps. Isotope Mass of the Isotope % abundance Product (as a decimal) Boron - 10 10. 013 . 199 Boron - 11 11. 009 . 801 Average Atomic Mass
- Slides: 10