Distinguishing Acids and Bases Acid donates H Base
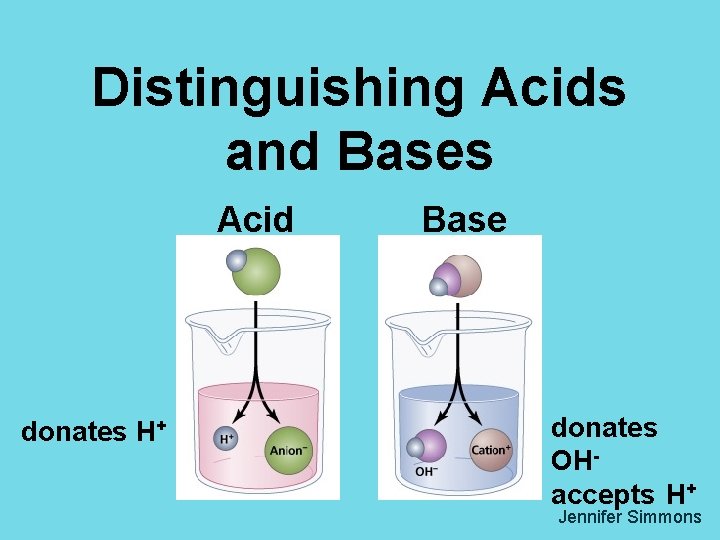
Distinguishing Acids and Bases Acid donates H+ Base donates OHaccepts H+ Jennifer Simmons
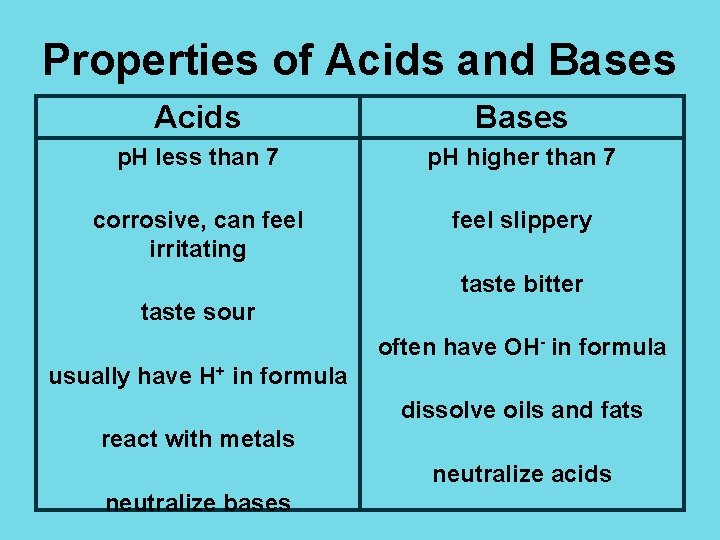
Properties of Acids and Bases Acids Bases p. H less than 7 p. H higher than 7 corrosive, can feel irritating feel slippery taste bitter taste sour often have OH- in formula usually have H+ in formula dissolve oils and fats react with metals neutralize acids neutralize bases
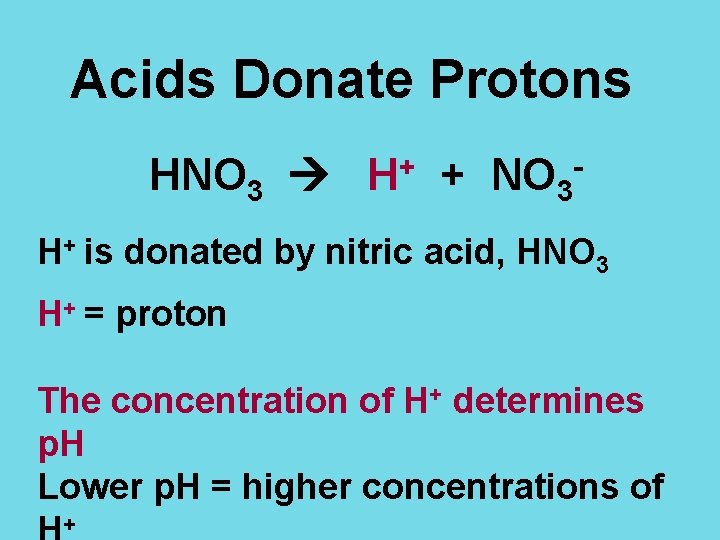
Acids Donate Protons HNO 3 H+ + NO 3 H+ is donated by nitric acid, HNO 3 H+ = proton The concentration of H+ determines p. H Lower p. H = higher concentrations of +
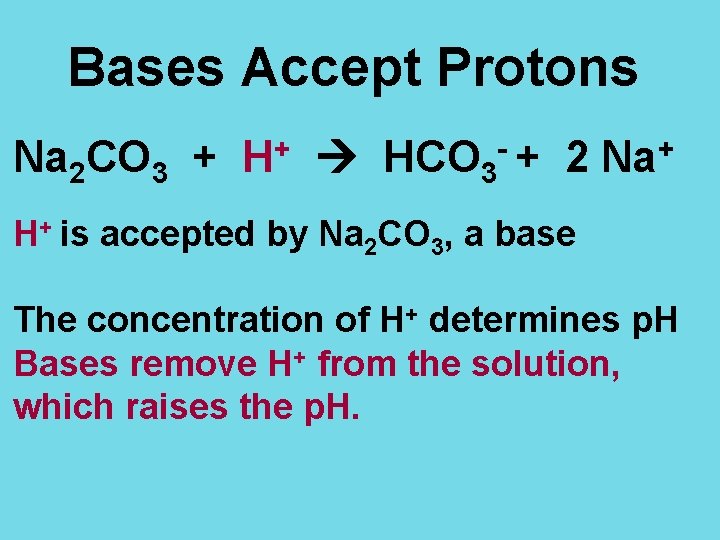
Bases Accept Protons Na 2 CO 3 + + H HCO 3 -+ 2 + Na H+ is accepted by Na 2 CO 3, a base The concentration of H+ determines p. H Bases remove H+ from the solution, which raises the p. H.
Formulas of Acids Since acids are H+ donors, their formulas can often be recognized by a leading H: HNO 3 H 2 CO 3 HCl H 2 SO 4 HCl. O nitric acid carbonic acid hydrochloric acid sulfuric acid chloric acid
Formulas of Bases that contain OH- can be recognized by their formula: Na. OH KOH Mg(OH)2 Ca(OH)2 Ba(OH)2 sodium hydroxide potassium hydroxide magnesium hydroxide calcium hydroxide barium hydroxide
Reactions with Indicators • Acids and bases react differently with chemicals known as indicators. • We can use indicators to identify whether a substance is an acid or a base. • There are 2 common indicators: ØLitmus ØPhenolphthalein
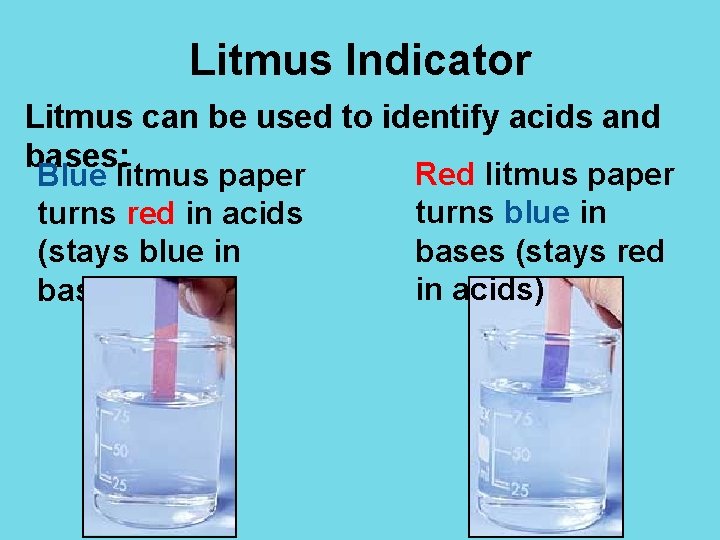
Litmus Indicator Litmus can be used to identify acids and bases: Red litmus paper Blue litmus paper turns blue in turns red in acids bases (stays red (stays blue in in acids) bases)
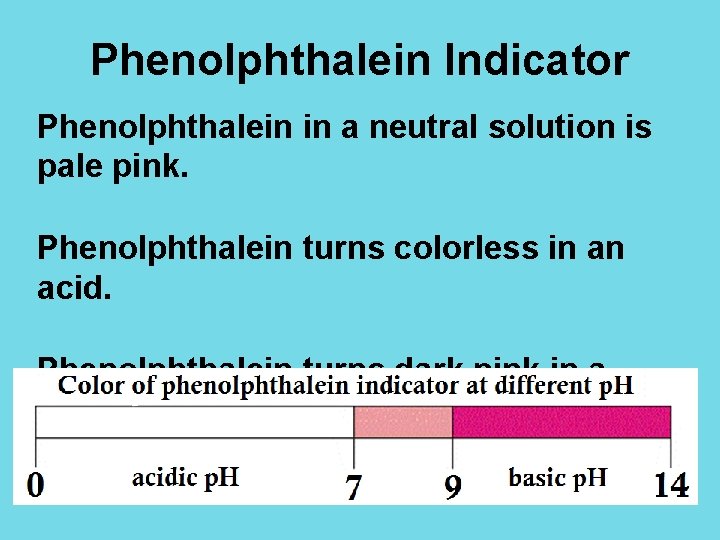
Phenolphthalein Indicator Phenolphthalein in a neutral solution is pale pink. Phenolphthalein turns colorless in an acid. Phenolphthalein turns dark pink in a base.
- Slides: 9