Distinct mechanisms regulate central vs peripheral tolerance self











- Slides: 36

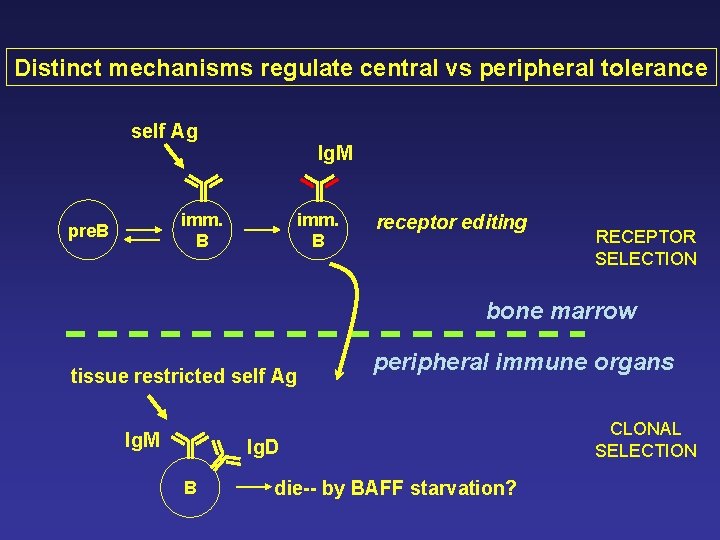
Distinct mechanisms regulate central vs peripheral tolerance self Ag Ig. M imm. B pre. B imm. B receptor editing RECEPTOR SELECTION bone marrow tissue restricted self Ag Ig. M peripheral immune organs Ig. D B die-- by BAFF starvation? CLONAL SELECTION

A fact that you may find surprising Most randomly generated antigen receptors are autoreactive • This is a consequence of the size/flexibility of antigen receptors that allow antibodies to cross react • Tolerance is a major problem for the immune system which is dealt with in a series of steps to reduce the frequency and affinity of self reactive cells

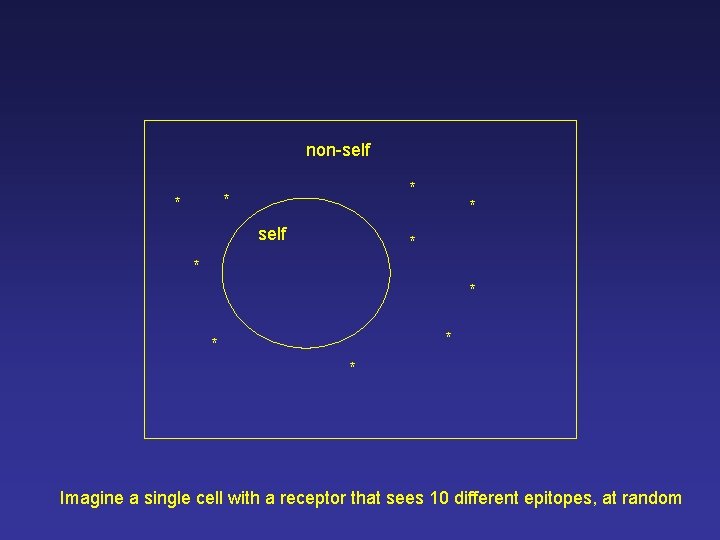
non-self * * * * * Imagine a single cell with a receptor that sees 10 different epitopes, at random

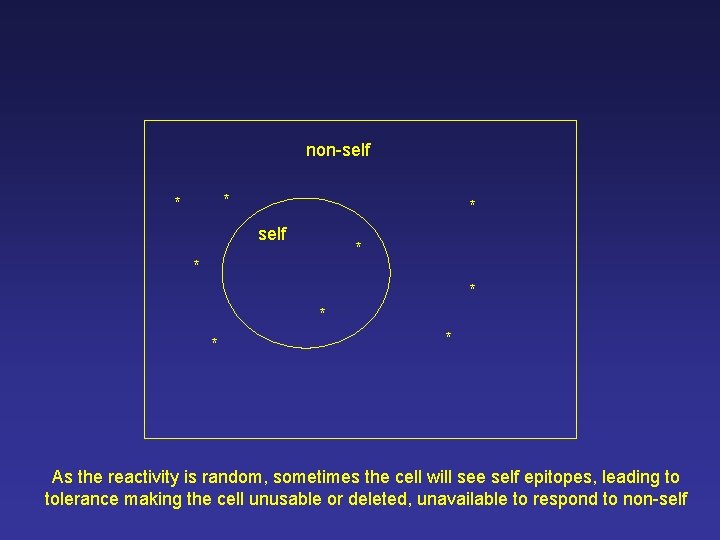
non-self * * * As the reactivity is random, sometimes the cell will see self epitopes, leading to tolerance making the cell unusable or deleted, unavailable to respond to non-self

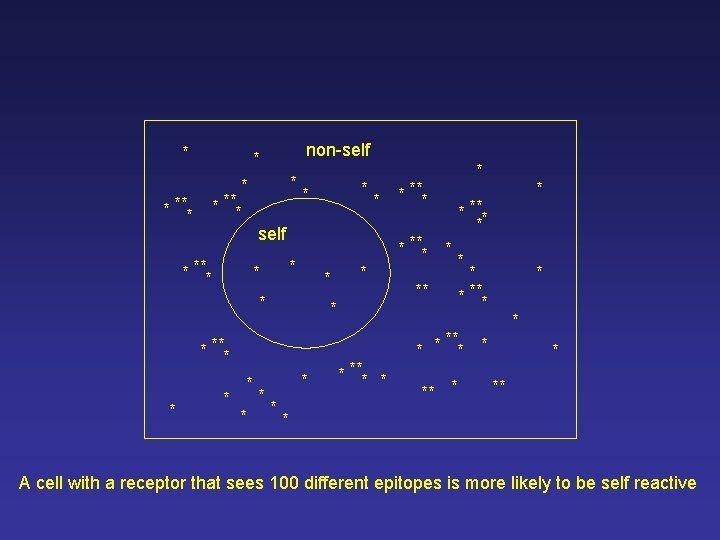
This cell is more useful! * non-self * * *** * * * self * * * *** * * *** * * * *** * ** * Imagine a single cell with a receptor that sees 100 different epitopes, at random

* non-self * * *** * self * *** * * * *** * * * *** * * * * ** * A cell with a receptor that sees 100 different epitopes is more likely to be self reactive

Because the ability to see foreign antigens increases linearly with antigen receptor cross reactivity, while the chance of a cell being eliminated by tolerance increases exponentially, there is an optimal extent of multireactivity that can be calculated. It turns out that at this optimum, which is independent of assumptions about the actual size of the self antigen repertoire, ~62% (1 -e-1) of cells should be autoreactive. However, the ability of cells to edit may allow the extent of cross reactivity to be even higher. Nemazee 1996 Immunol. Today 17: 25

In the bone marrow Self antigen Receptor-less cell (pre B) Innocuous receptor Autoreactive receptor Recombinase ON Recombinase OFF Recombinase ON

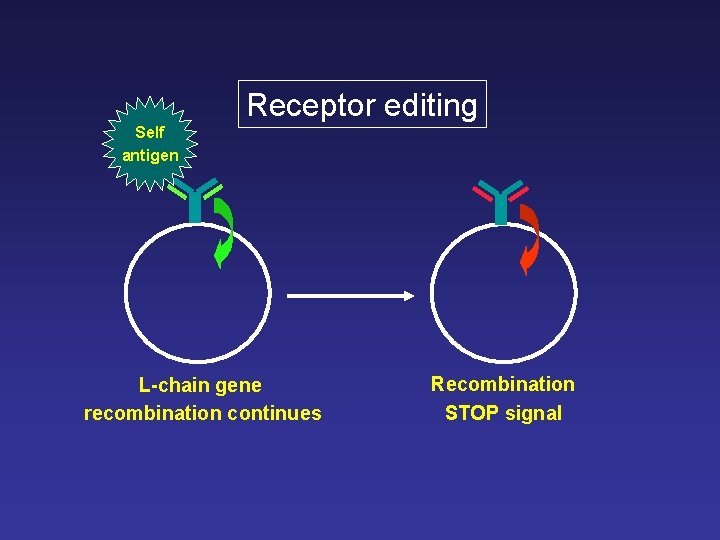
Receptor editing Self antigen L-chain gene recombination continues Recombination STOP signal

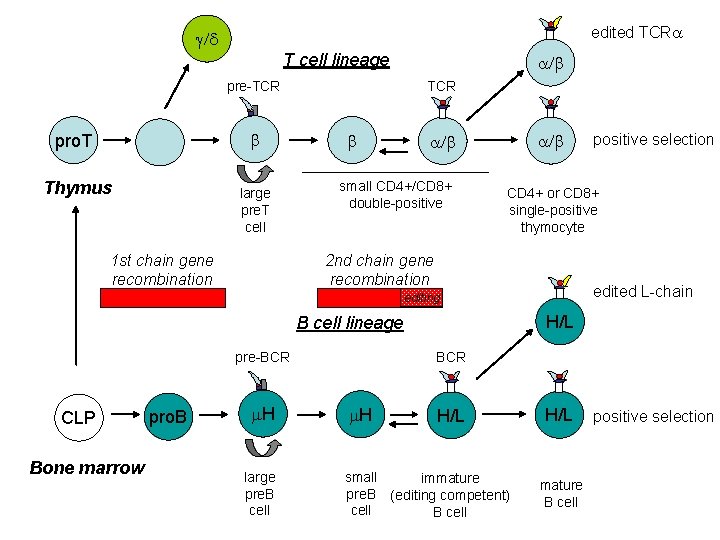
edited TCRa g/d T cell lineage pre-TCR b pro. T Thymus large pre. T cell 1 st chain gene recombination a/b TCR b a/b small CD 4+/CD 8+ double-positive CD 4+ or CD 8+ single-positive thymocyte 2 nd chain gene recombination edited L-chain editing H/L B cell lineage pre-BCR CLP Bone marrow pro. B m. H large pre. B cell positive selection BCR m. H H/L small immature pre. B (editing competent) cell B cell H/L mature B cell positive selection

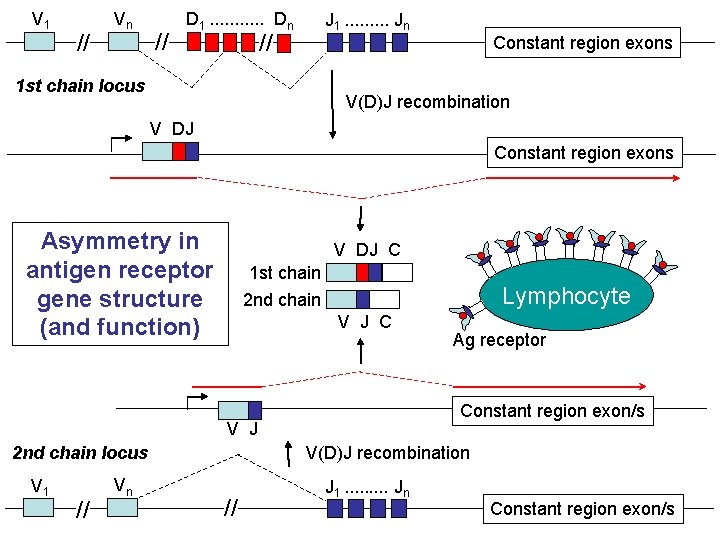
V 1 // Vn // D 1. . . Dn J 1. . Jn // 1 st chain locus Constant region exons V(D)J recombination V DJ Constant region exons Asymmetry in antigen receptor gene structure (and function) V DJ C 1 st chain Lymphocyte 2 nd chain V J C Constant region exon/s V J V(D)J recombination 2 nd chain locus V 1 // Vn Ag receptor // J 1. . Jn Constant region exon/s

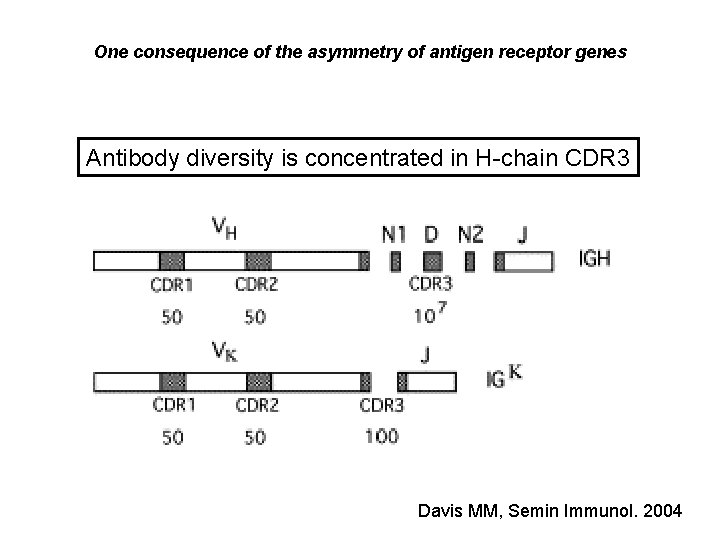
One consequence of the asymmetry of antigen receptor genes Antibody diversity is concentrated in H-chain CDR 3 Davis MM, Semin Immunol. 2004

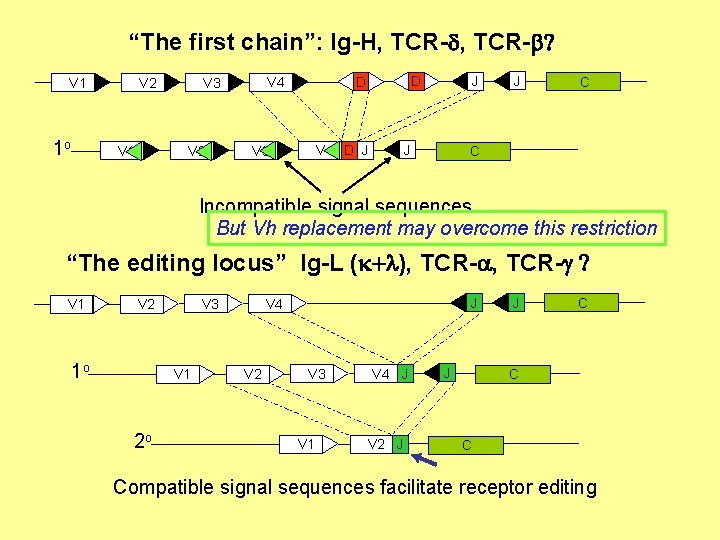
“The first chain”: Ig-H, TCR-d, TCR-b? V 1 1 o V 1 V 2 J V 4 D J V 3 J D D V 4 V 3 V 2 J C C Incompatible signal sequences But Vh replacement may overcome this restriction “The editing locus” Ig-L (k+l), TCR-a, TCR-g ? V 1 1 o V 1 2 o J V 4 V 3 V 2 V 3 V 1 V 4 J V 2 J J J C Compatible signal sequences facilitate receptor editing

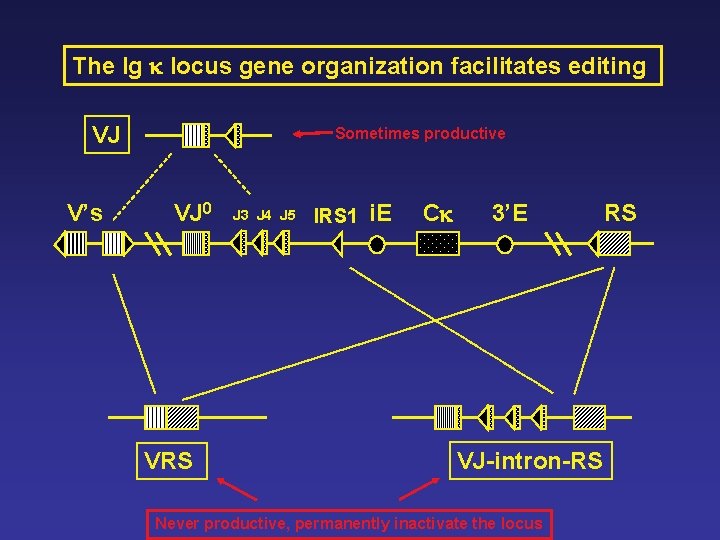
The Ig k locus gene organization facilitates editing VJ V’s Sometimes productive VJ 0 VRS J 3 J 4 J 5 IRS 1 i. E Ck 3’E VJ-intron-RS Never productive, permanently inactivate the locus RS

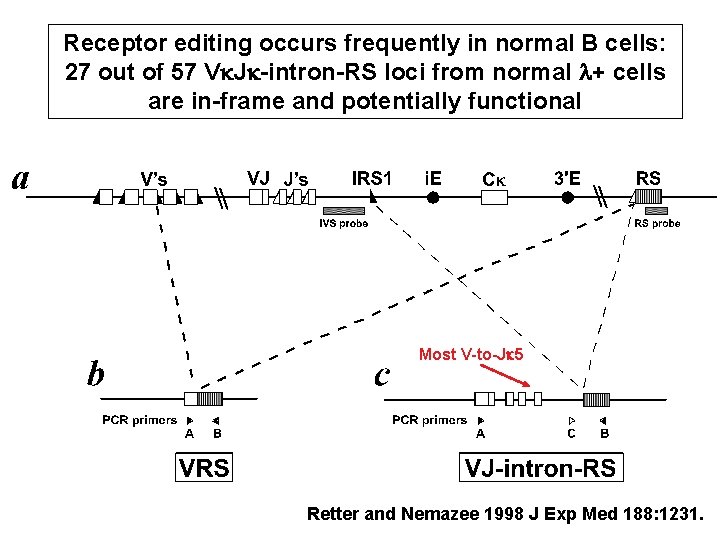
Receptor editing occurs frequently in normal B cells: 27 out of 57 Vk. Jk-intron-RS loci from normal l+ cells are in-frame and potentially functional Most V-to-Jk 5 Retter and Nemazee 1998 J Exp Med 188: 1231.

Evidence for the high likelihood of a cell to be autoreactive -Evidence of editing on the Igk locus in a large fraction of Igl B cells (Retter and Nemazee 1998 J Exp Med 188: 1231; Brauninger 2001 EJI) -A dearth of arginines in antibody combining sites, except in disease- there appears to be ~40% loss in B cells because of this selection pressure alone (Louzoun et al. 2002 Semin. Immunol. 14: 239) -A decreasing frequency of multireactive B cells with developmental progression, starting with 75% at the bone marrow stage to less than 30% at later stages (Wardemann et al 2003, Science 301: 1374 -Random Ig H/L transgene pairs are often autoreactive and lead to editing in vivo (Novobrantseva et al. 2005. Int. Immunol. 17: 343 )

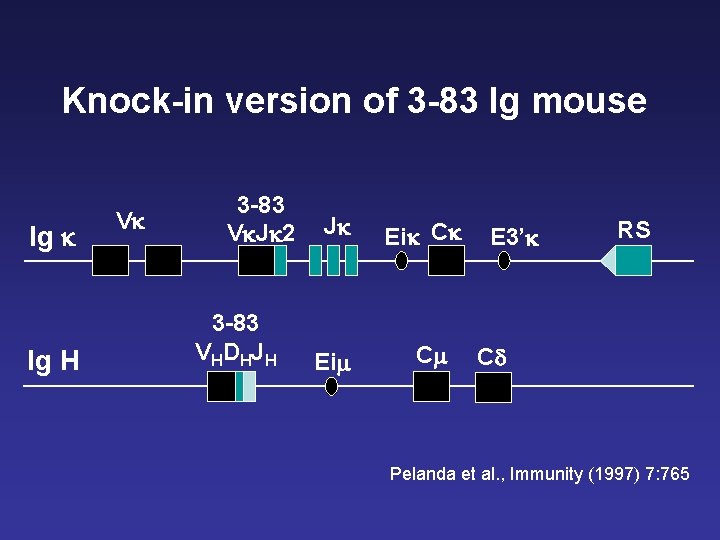
Knock-in version of 3 -83 Ig mouse Ig k Ig H Vk 3 -83 Vk. Jk 2 3 -83 V HD HJ H Jk Eim Eik Ck Cm E 3’k RS Cd Pelanda et al. , Immunity (1997) 7: 765


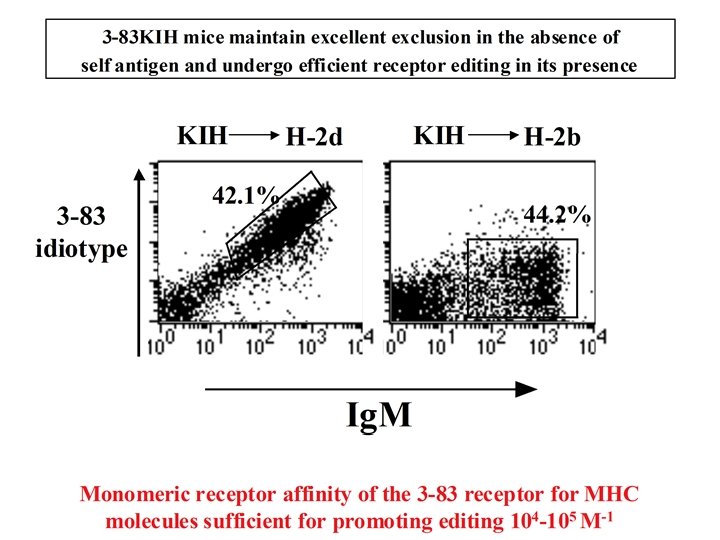
Summary -Most generated B cells are autoreactive. -Most immature B cells are editing-competent. -Editing has a low threshold affinity in anti-MHC Tg (3 -83). -Editing can occur often under normal conditions. -Tolerance is likely to be the major inducer of editing.

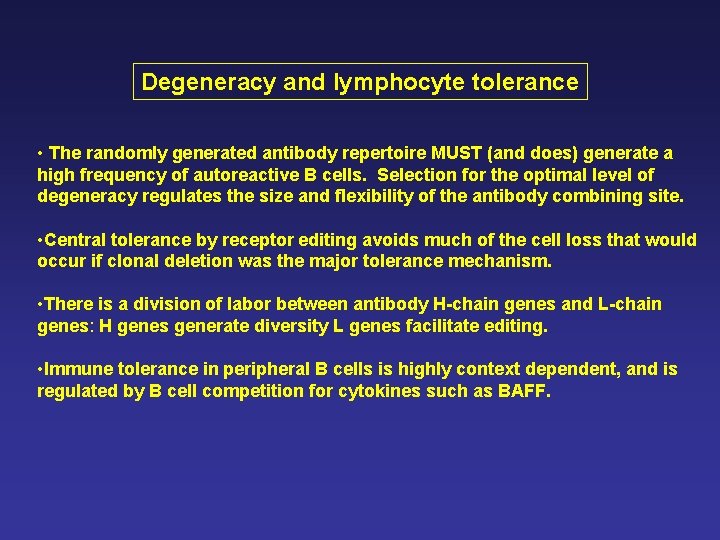
Degeneracy and lymphocyte tolerance • The randomly generated antibody repertoire MUST (and does) generate a high frequency of autoreactive B cells. Selection for the optimal level of degeneracy regulates the size and flexibility of the antibody combining site. • Central tolerance by receptor editing avoids much of the cell loss that would occur if clonal deletion was the major tolerance mechanism. • There is a division of labor between antibody H-chain genes and L-chain genes: H genes generate diversity L genes facilitate editing. • Immune tolerance in peripheral B cells is highly context dependent, and is regulated by B cell competition for cytokines such as BAFF.

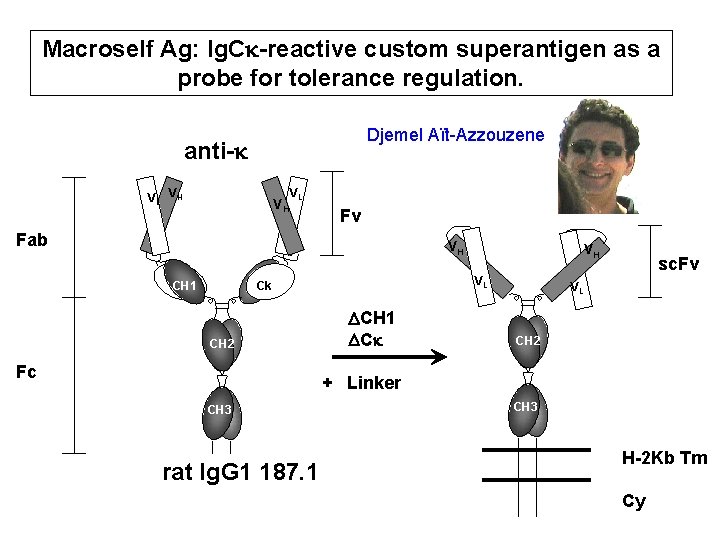
Macroself Ag: Ig. Ck-reactive custom superantigen as a probe for tolerance regulation. Djemel Aït-Azzouzene anti-k VL VH VH VL Fv Fab VH CH 1 VL Ck CH 2 Fc VH DCH 1 DCk sc. Fv VL CH 2 + Linker CH 3 rat Ig. G 1 187. 1 CH 3 H-2 Kb Tm Cy

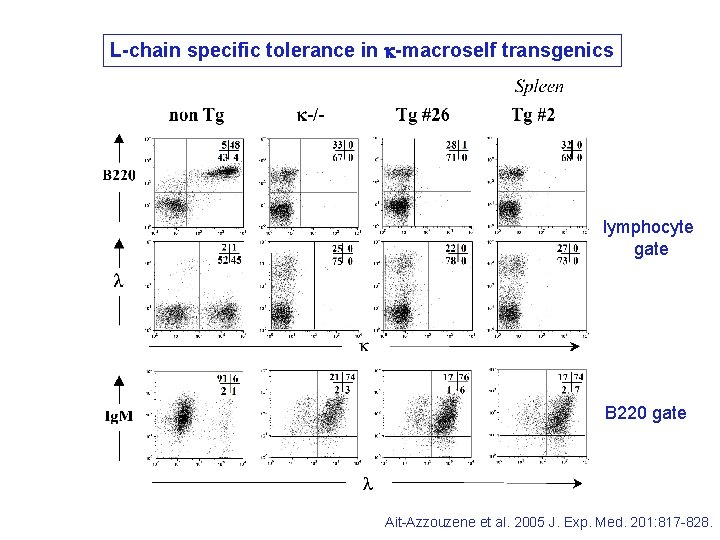
L-chain specific tolerance in k-macroself transgenics lymphocyte gate B 220 gate Ait-Azzouzene et al. 2005 J. Exp. Med. 201: 817 -828.

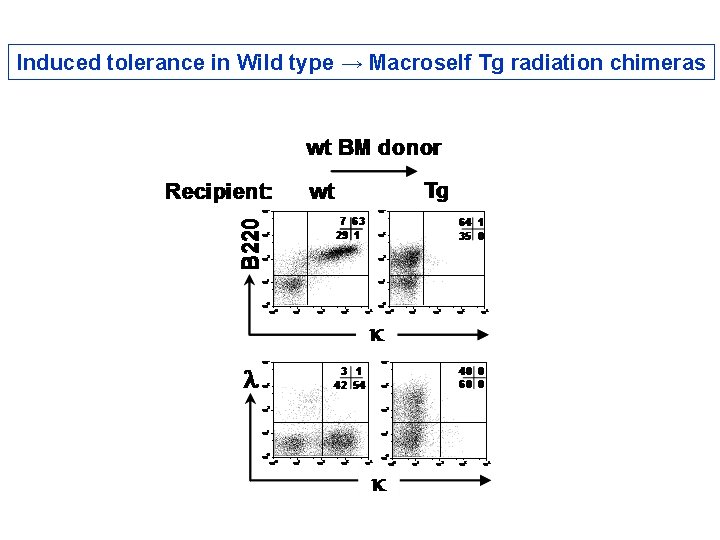
Induced tolerance in Wild type → Macroself Tg radiation chimeras

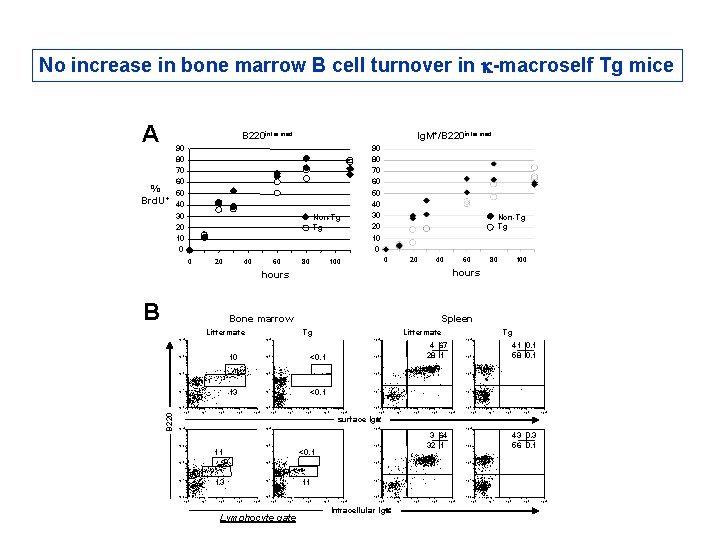
No increase in bone marrow B cell turnover in k-macroself Tg mice A B 220 intermed Ig. M+/B 220 intermed 90 80 70 60 % 50 Brd. U+ 40 30 20 10 0 90 80 70 60 50 40 30 20 10 0 Non-Tg Tg 0 20 40 60 80 Non-Tg Tg 0 100 20 40 hours B Bone marrow Littermate 10 13 <0. 1 10 0 10 1 10 2 10 3 10 4 Tg 10 1 10 2 10 3 10 2 10 1 10 4 41 0. 1 58 0. 1 10 3 10 0 100 10 4 4 67 28 1 10 3 10 2 10 1 B 220 10 4 10 3 10 2 10 0 Littermate 10 4 10 3 80 Spleen Tg 10 4 60 hours 10 0 10 1 10 2 10 3 10 4 surface Igk 10 4 10 3 11 10 2 13 10 1 10 0 <0. 1 10 2 11 10 0 10 1 10 2 10 3 10 4 3 64 32 1 10 2 10 1 10 0 10 1 Lymphocyte gate 10 2 10 3 10 4 10 0 Intracellular Igk 10 1 10 2 10 3 43 0. 3 56 0. 1 10 3 10 4 10 0 10 1 10 2 10 3 10 4

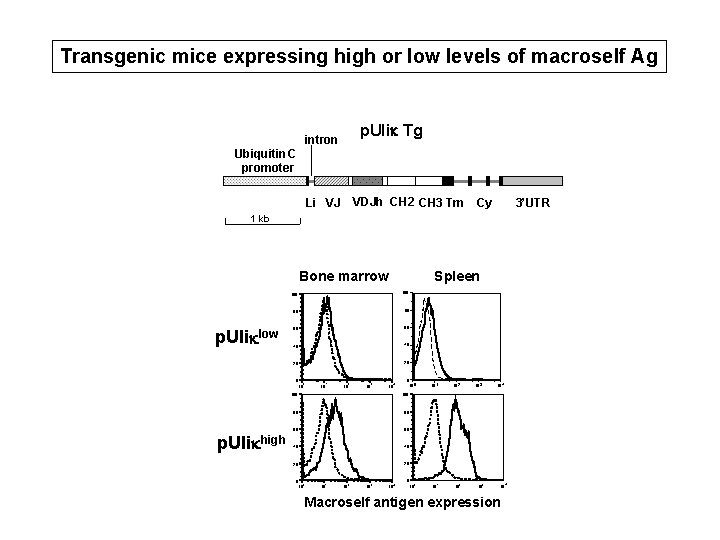
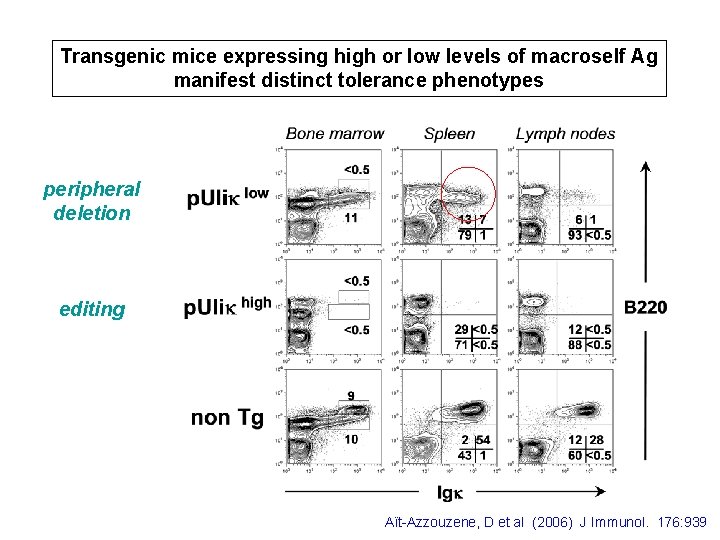
Transgenic mice expressing high or low levels of macroself Ag p. Ulik Tg intron Ubiquitin C promoter Li VJ VDJh CH 2 CH 3 Tm Cy 3’UTR 1 kb Bone marrow p. Uliklow Spleen 100 80 80 60 60 40 40 20 20 0 0 10 p. Ulikhigh 0 10 1 10 2 10 3 10 4 10 0 10 10 100 80 80 60 60 40 40 20 20 0 10 1 10 2 10 3 10 4 1 10 2 10 3 3 10 10 4 10 Macroself antigen expression 4

Transgenic mice expressing high or low levels of macroself Ag manifest distinct tolerance phenotypes peripheral deletion editing Aït-Azzouzene, D et al (2006) J Immunol. 176: 939

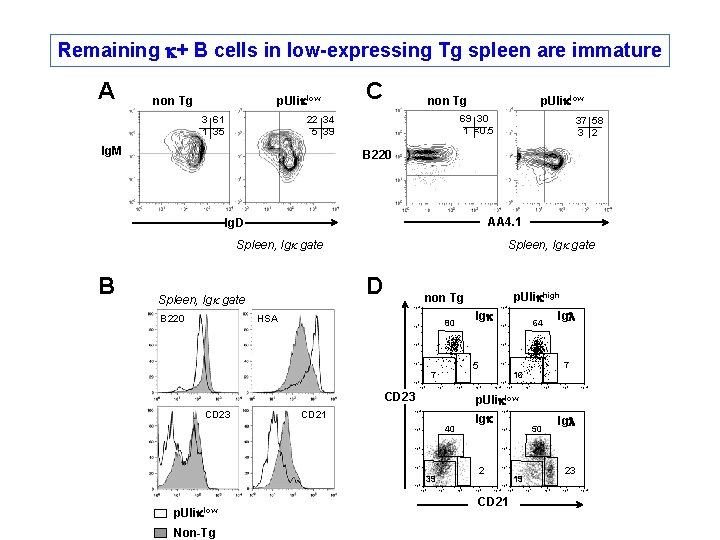
Remaining k+ B cells in low-expressing Tg spleen are immature A non Tg p. Uliklow 3 61 1 35 C non Tg 69 30 1 <0. 5 22 34 5 39 Ig. M p. Uliklow 37 58 3 2 B 220 AA 4. 1 Ig. D Spleen, Igk gate B 220 Spleen, Igk gate p. Ulikhigh non Tg 10 4 HSA Igk 80 10 3 10 4 10 2 5 10 1 7 16 10 0 10 1 10 2 CD 23 CD 21 10 3 10 4 10 1 10 2 10 3 10 4 Igk 40 10 2 10 1 10 0 Igl 50 10 3 10 2 10 1 2 39 10 0 Non-Tg 10 0 p. Uliklow 10 4 10 3 p. Uliklow 7 10 1 10 0 CD 23 Igl 64 10 3 10 0 10 1 10 2 10 3 10 4 10 0 CD 21 23 19 10 1 10 2 10 3 10 4

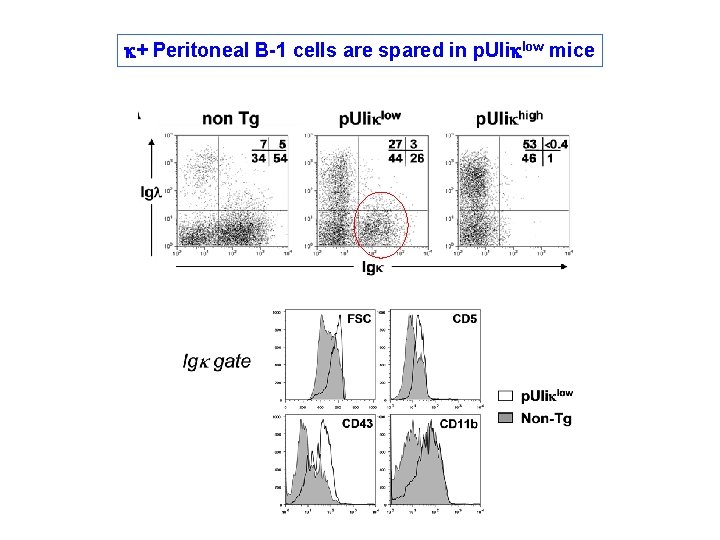
k+ Peritoneal B-1 cells are spared in p. UIiklow mice

Peritoneal k+ cells of p. UIiklow mice are not short-lived

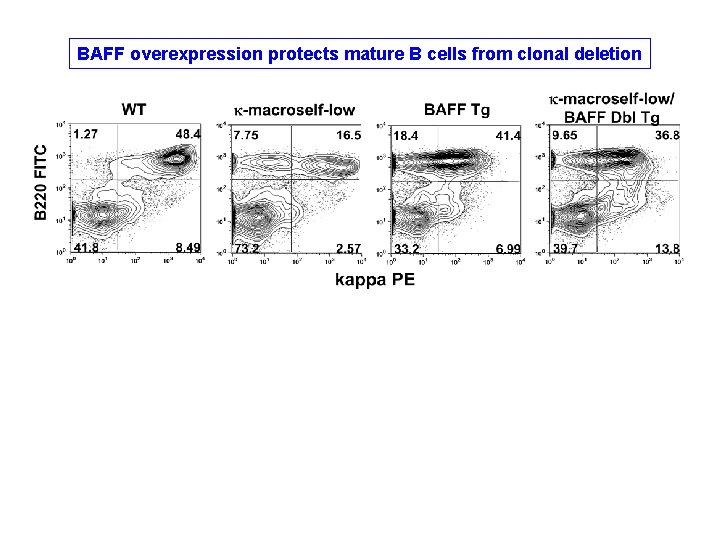
BAFF overexpression protects mature B cells from clonal deletion

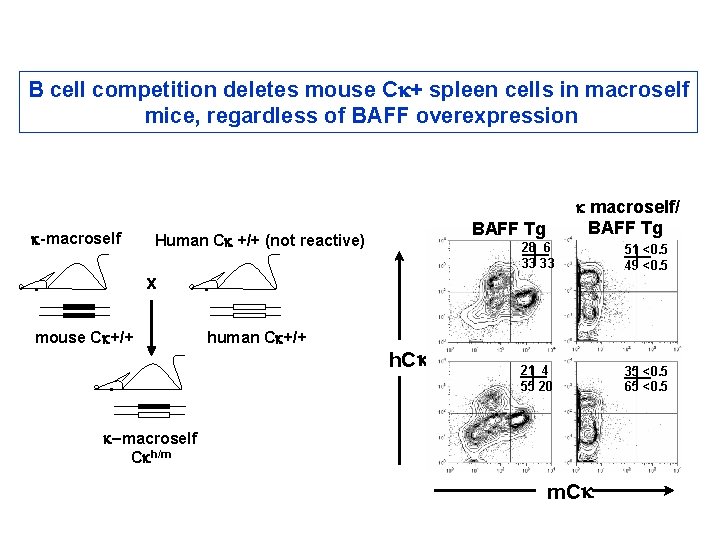
B cell competition deletes mouse Ck+ spleen cells in macroself mice, regardless of BAFF overexpression k-macroself . BAFF Tg Human Ck +/+ (not reactive) x mouse Ck+/+ . . human Ck+/+ k macroself/ BAFF Tg h. C k 28 6 33 33 51 <0. 5 49 <0. 5 21 4 55 20 35 <0. 5 65 <0. 5 k-macroself Ckh/m m. C k

SUMMARY Excess BAFF can partly suppress peripheral deletion provided that competition from non-autoreactive B cells is minimal (i. e. , 6%, not 50%)

B-1 cells in the peritoneum are also eliminated by low level superantigen when competing B cells are present

Tolerance steps in the preimmune repertoire 13 14 1 16 15 4 3 2 17 5 19 20 18 6 Receptor editing in bone marrow Generated repertoire 9 8 7 Tolerance 13 1 15 14’ 3 2 16’ 4 5 6 7’ 12 Edited repertoire 20’ 19 18 17’ 11 10 8 9 10 12 Tolerance Deletion in the peripheral immune tissue 13 1 15 14’ 2 3 16’ 4 Peripherally tolerized repertoire 17’ 5 6 10 12

In the peripheral lymphoid tissues Autoreactivity of increasing affinity 1 2 3 4 5 6 7 8 9 10 11 12

The Scripps Research Institute Amanda Gavin Bao Duong José Luis Vela Christoph Huber Takayuki Ota Patrick Skog Min Lim Acknowledgments Kurt Bürki Roberta Pelanda/ K. Rajewsky Michel Nussenzweig Funding: NIAID, NIGMS David Russell Susan Tiegs Julie Lang Valérie Kouskoff Marc Retter Laurent Verkoczy Djemel Aït-Azzouzene Discussion points: • Tolerance is a fact • Editing regulates antigen receptor gene stru • Size of the antibody combining site • Why antigen receptors aren't single chain • Retention of k/l • Role of competition in regulating tolerance