Dissolving Process Polarity The polarity of the solute





- Slides: 7

Dissolving Process Polarity – The polarity of the solute and solvent has a great impact on whether they will dissolve – Like dissolves like (polar dissolves polar) – Miscible – two or more liquids that are able to dissolve into each other in various proportions. – Immiscible – two or more liquids that do not mix with each other.

How to dissolve immiscible liquids A compound that concentrates at the boundary surface between two immiscible phases. It has a polar end a nonpolar end. Surfactant Example is a detergent Any mixture of two or more immiscible liquids in which one liquid is dispensed in the other. Emulsion

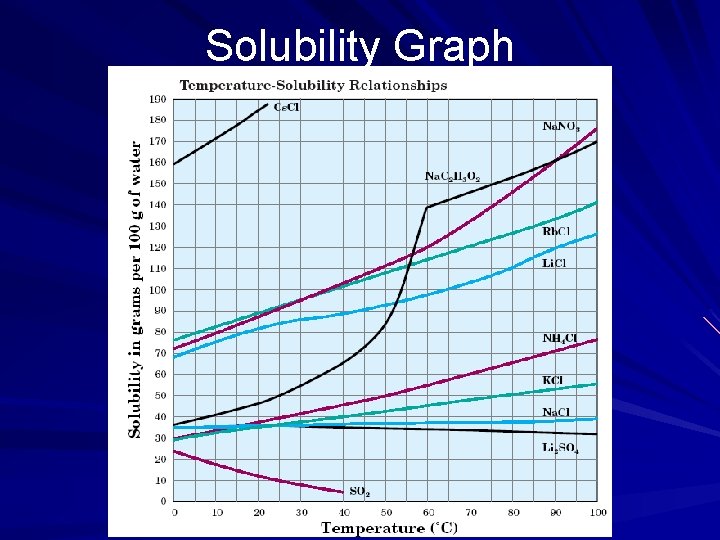
Dissolving Process Surface area – Smaller the surface area, the faster the solute will dissolve Agitation (stirring) increases the rate of dissolving Temperature – Temperature affects solubility – For solids and liquids, increase temperature = increase in solubility – When gases are involved, an increase in temperature = decrease in solubility.

Solubility Graph

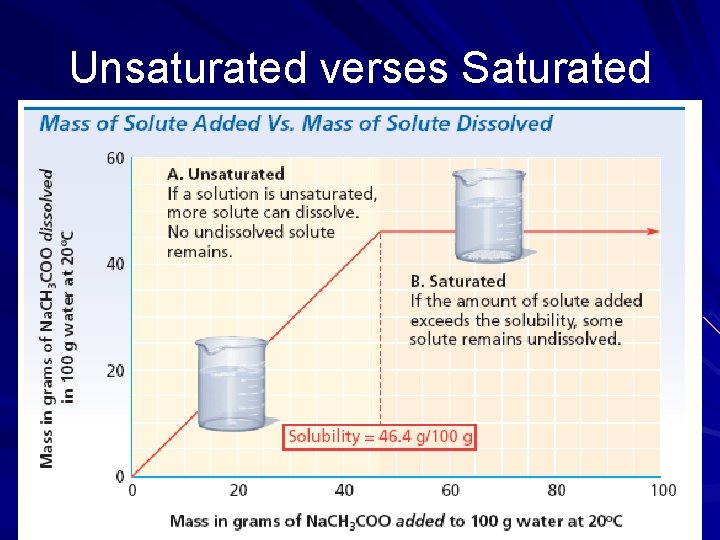
Saturation A solution that cannot dissolve any more solute under the current conditions. Saturated solution A solution that contains less solute than a saturated solution and is able to dissolve additional solute. Unsaturated solution A solution that contains more solute than what is required to reach equilibrium at a given temperature Supersaturated solution

Unsaturated verses Saturated

Henry’s Law The law that states that at constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas on the surface of the liquid. The higher the pressure of the gas, the higher its solubility. Brownian Motion The random movement of microscopic particles suspended in a fluid.