Dissolving and evaporating Aseel Samaro Introduction Gemstones such









- Slides: 14

Dissolving and evaporating Aseel Samaro

Introduction § Gemstones, such as amethyst, are crystal that formed deep in the Earth’s crust. § Water dissolved salts and as this solution cooled over thousands of years, the precious crystals formed.

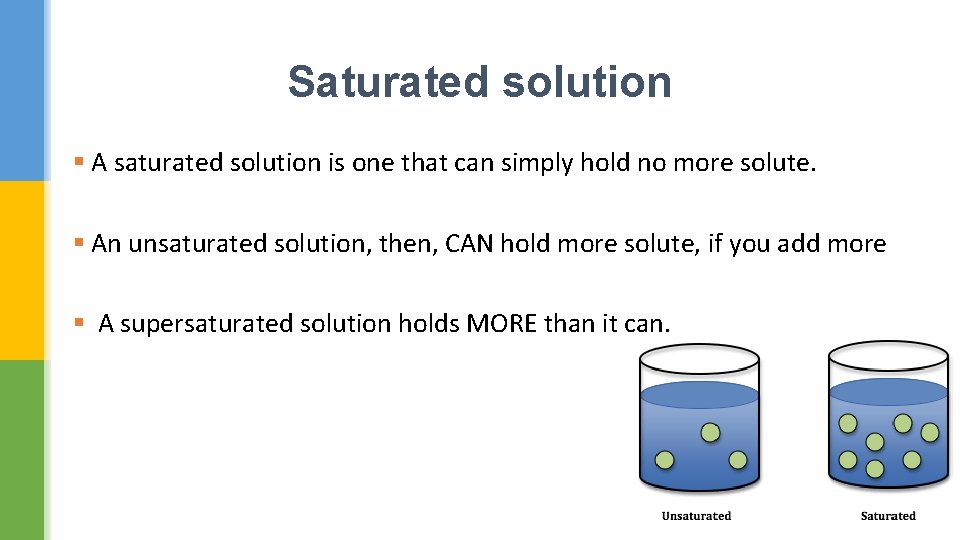
Saturated solution § A saturated solution is one that can simply hold no more solute. § An unsaturated solution, then, CAN hold more solute, if you add more § A supersaturated solution holds MORE than it can.

Temperature effects § The mass of solute that dissolves in a solvent at a particular temperature is called its solubility. § One way to help things dissolve is to increase the temperature of the water. § This is why we wash clothes in warm water. § Any soluble stains in the clothes will dissolve better at a higher temperature.

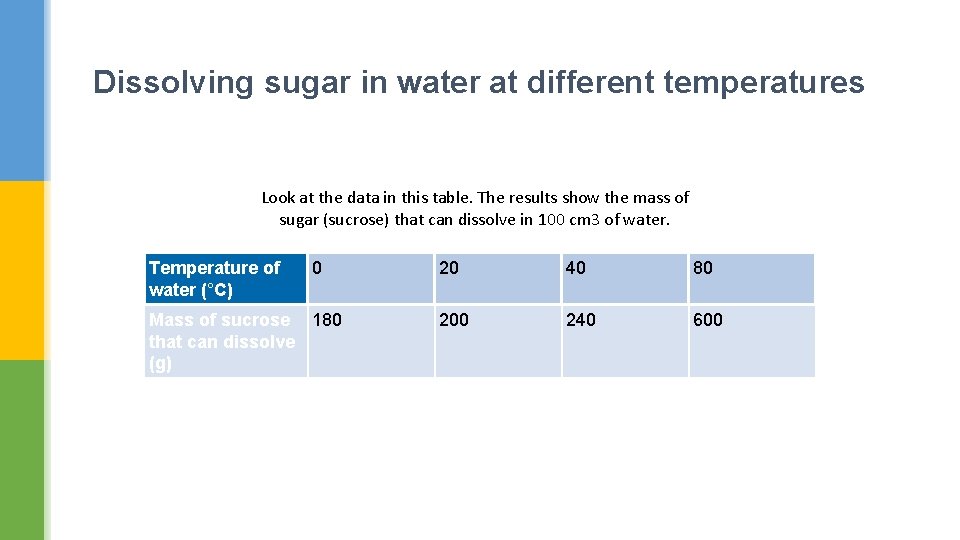
Dissolving sugar in water at different temperatures Look at the data in this table. The results show the mass of sugar (sucrose) that can dissolve in 100 cm 3 of water. Temperature of water (°C) 0 Mass of sucrose 180 that can dissolve (g) 20 40 80 200 240 600

What does the data in the previous table tell you about the solubility of sucrose at different temperatures?

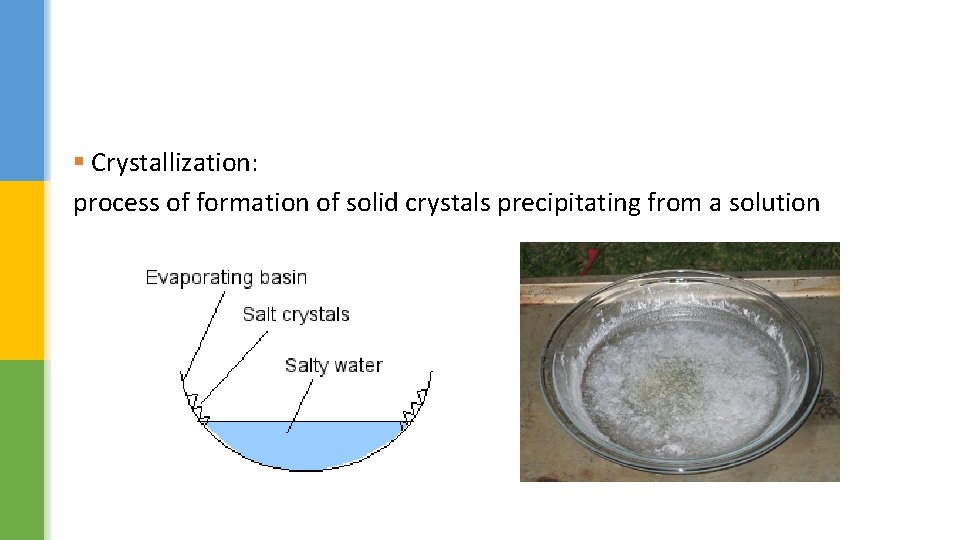
Making crystals § Heat can also be used to separate soluble substances from their solutions. § When the solvent evaporates it leaves behind the solid solute – this is called crystallisation. § If this process happens quickly, small crystals of the solute will form. § However, if the evaporation happens slowly, bigger crystals can grow.

§ Crystallization: process of formation of solid crystals precipitating from a solution

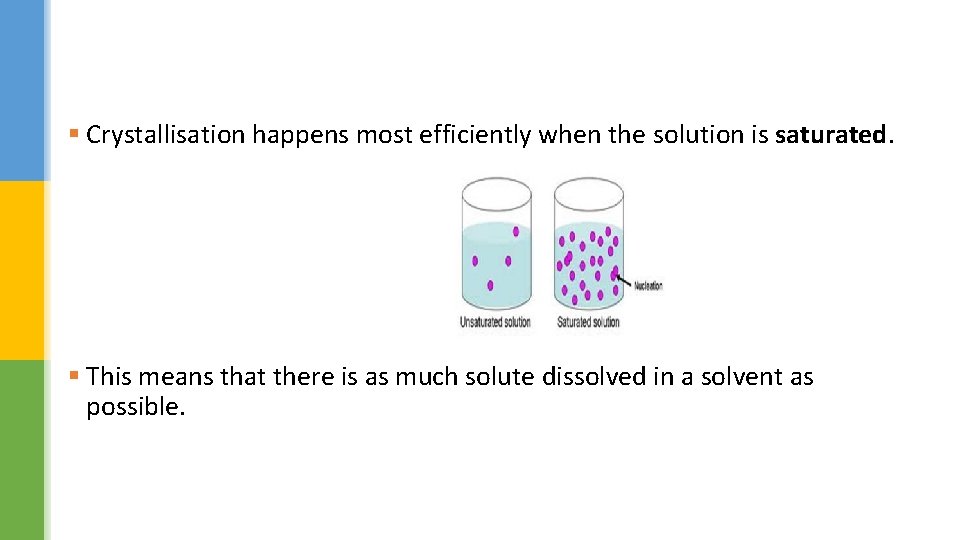
§ Crystallisation happens most efficiently when the solution is saturated. § This means that there is as much solute dissolved in a solvent as possible.

§ The solubility of substances depends on the temperature of the solvent. § If any more solute is added to a saturated solution it will not dissolve. § As the solvent cools, the crystals start to form. (As the solution cools, the solvent can no longer hold all of the solute molecules, and they begin to leave the solution and form solid crystals)

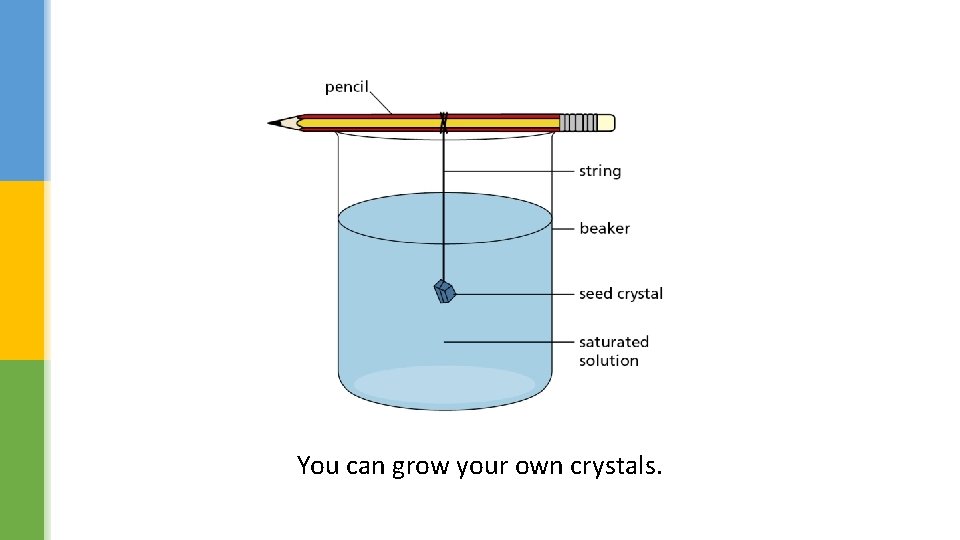
You can grow your own crystals.

What is a ‘saturated’ solution? One in which no more solute will dissolve. Describe how you could grow salt crystals. Stages should include preparation of saturated solution and evaporation of water to grow crystals. Why do you think that the crystals start to form as the solvent cools? Less solute can be dissolved at lower temperatures/less soluble

Using graphs § Referring to the figure in your book: § Substances dissolve more in hot water because the water molecules have more energy and move faster. § They can break down the crystals and separate the solute molecules more quickly. § Solubility also depends on the type of solute.

HOMEWORK