Dissolution where physiochemistry meets biology Phys Chem Forum

Dissolution – where physiochemistry meets biology Phys. Chem Forum, 20 th Sept 2011 Nottingham Dr Brian Henry Pharmaceutical Science Pfizer Global Research and Development brian. henry@pfizer. com

Today’s Talk Biology Physiochemistry 2
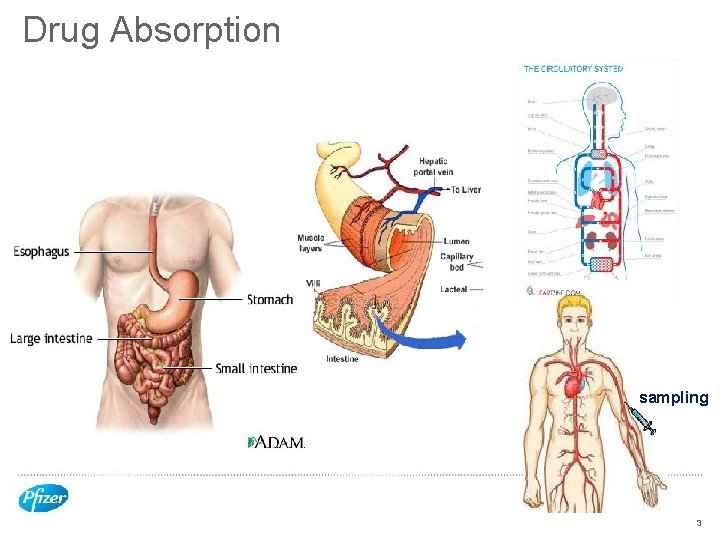
Drug Absorption sampling 3
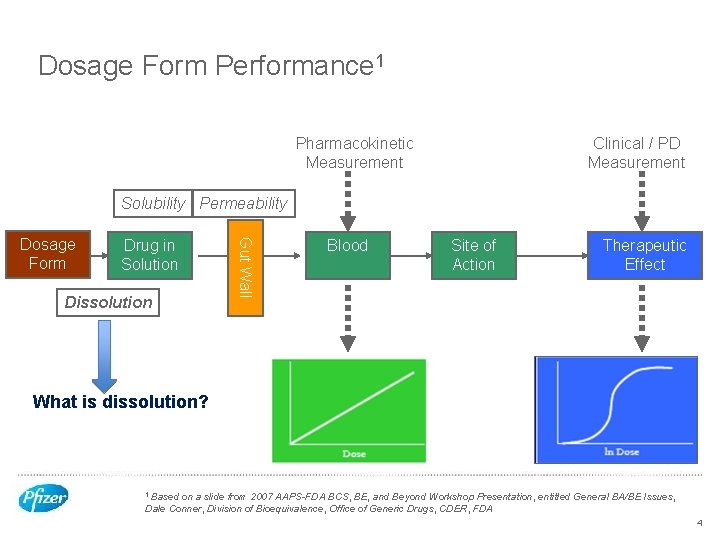
Dosage Form Performance 1 Pharmacokinetic Measurement Clinical / PD Measurement Solubility Permeability Drug in Solution Dissolution Gut Wall Dosage Form Blood Site of Action Therapeutic Effect What is dissolution? 1 Based on a slide from 2007 AAPS-FDA BCS, BE, and Beyond Workshop Presentation, entitled General BA/BE Issues, Dale Conner, Division of Bioequivalence, Office of Generic Drugs, CDER, FDA 4

Dissolution Testing of Oral Dosage Form What is Dissolution? • Dissolution is the rate at which a substance dissolves in a fluid. • In pharmaceutical practice, dissolution is the rate at which a drug in a dosage form dissolves into the fluid surrounding it. • In the case of modified release dosage forms dissolution rate and release rate of drug are controlled by the design of drug product 5
Two Very Different Purposes of Dissolution Test As a quality control measure for dosage form • Batch to batch reproducibility to assure consistency in quality of manufactured product • Shelf life stability • Assure manufacturing process changes do not impact performance (typically requires BE study) To predict dosage form PK performance in vivo • Guide formulation selection, design and scale-up during development – Quality by design and support regulatory filings • Help select and set specifications for API form and particle size • Guide bioequivalence strategy 6
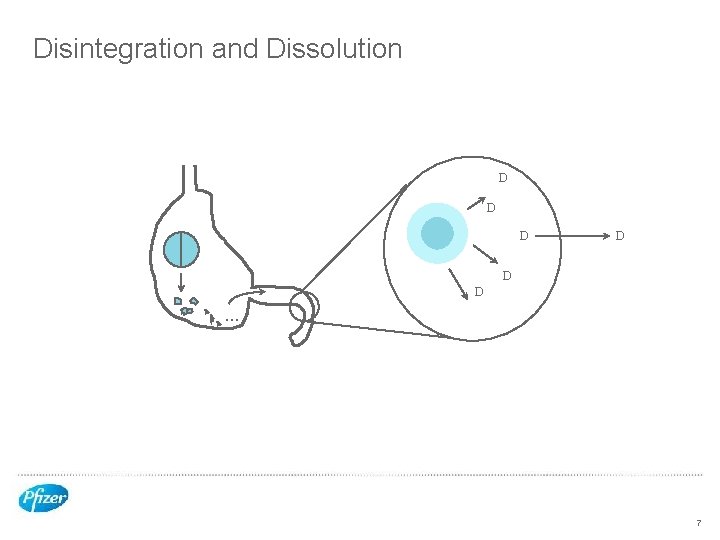
Disintegration and Dissolution D D D . . . 7

Dissolution Model Noyes and Whitney Equation Diffusion Layer Cs • M: the mass of solute dissolved at time t Solid Surface Bulk solution C x=0 x=h • d. M/dt: the mass rate of dissolution • D: diffusion coefficient of the solute in solution • S: the surface area of the exposed solid • h: the thickness of the diffusion layer • Cs: the solubility of the solid • C: the concentration of solute in the bulk solution and at time t 8

What Factors Influence Dissolution? The properties of drug • • • Solubility of the API in the dissolution medium Whether the API is hydrophilic or hydrophobic (ease of surface wetting) The particle size/shape of the API Whether the API is crystalline or amorphous in the drug product If there are polymorphs, which polymorph is present If a salt form is used The quality and design of the drug product • The composition of the drug product and how they are added • Manufacturing processes and steps • Whether the product is designed to immediately release the API, to delay release, or to release the drug over time. The condition of dissolution tested 9

USP Dissolution apparatus USP I/II USP IV 10

Dissolution of different 250 mg Crizotinib dosage forms 11
Crizotinib ‘Powder in Capsule’ vs Tablet BA study 12
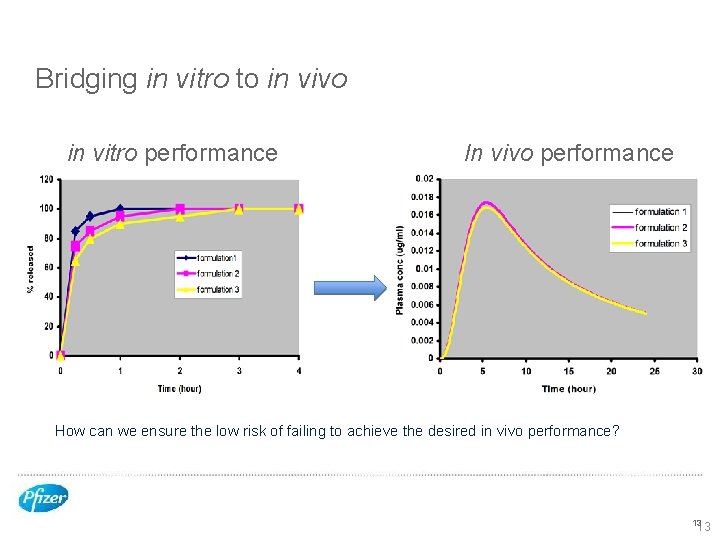
Bridging in vitro to in vivo in vitro performance In vivo performance How can we ensure the low risk of failing to achieve the desired in vivo performance? 13 13

In vitro/In vivo correlations (IVIVC) Definition 5 Definition A predictive mathematical treatment describing the relationship between an in vitro property of a dosage form (usually the rate or extent of drug release) and a relevant in vivo response (e. g. drug concentration in plasma or amount of drug absorbed). … to accurately and precisely predicting expected bioavailability characteristics for an ER product from dissolution profile characteristics … Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/ In Vivo Correlations, September 1997 5 14 14
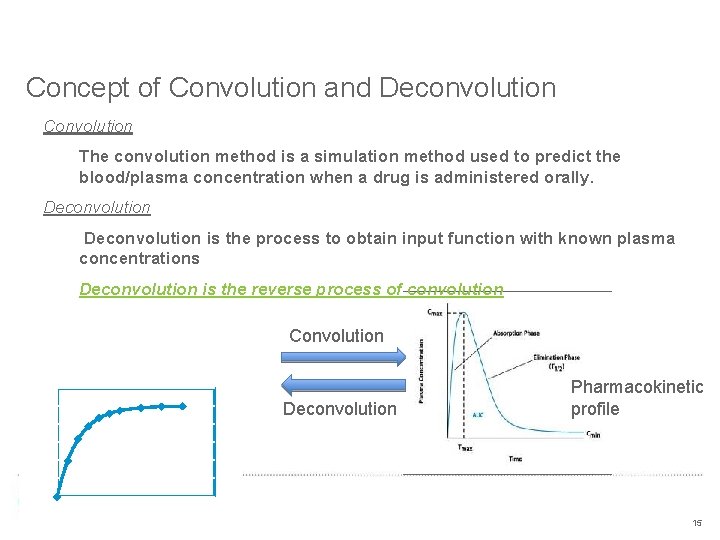
Concept of Convolution and Deconvolution Convolution The convolution method is a simulation method used to predict the blood/plasma concentration when a drug is administered orally. Deconvolution is the process to obtain input function with known plasma concentrations Deconvolution is the reverse process of convolution Convolution % drug absorbed 120 In vivo dissolution 100 Deconvolution 80 Pharmacokinetic profile 60 40 20 0 0 5 10 Time (hrs) 15 15

IVIVC Model 16 16

So that’s about it for dissolution, Dinner? …well, it’s rarely that straight forward 17

The problems with predicting dissolution are very fundamental Consider what is happening at the primary particle surface Surface area Diffusion Layer Particle size distribution Particle shape Wetting and aggregation Bulk solution solubility Cs p. H differences/precipitation Bile solubilisation Solid Surface Bulk solution C x=0 x=h Dissolution vs absorption rates Unstirred Water layer p. H gradient Bile micelle migration Mix hydrodynamics 18

Intrinsic dissolution rate; the simplest form of dissolution testing • Constant surface area compact (ensure solid form remains intact after compression) • Rotating disk (or static disk & rotating fluid) – Well defined hydrodynamics • Detection methodology (on-line or off line) • Temperature controlled • Means to assess solid form after experiment 19
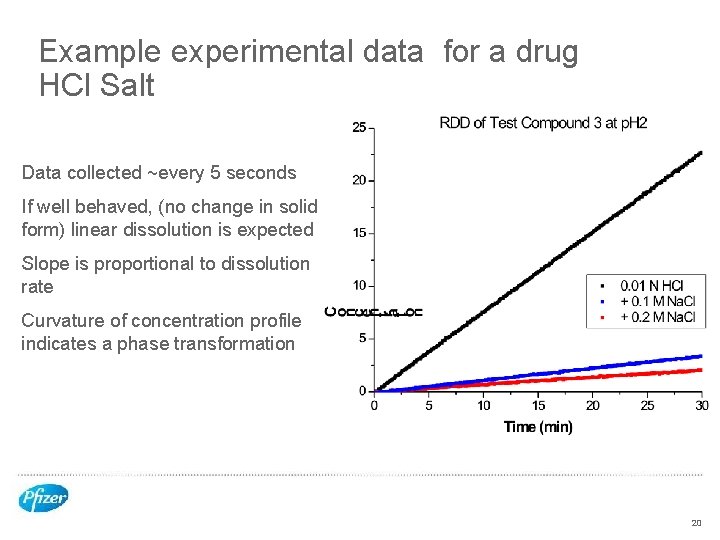
Example experimental data for a drug HCl Salt Data collected ~every 5 seconds If well behaved, (no change in solid form) linear dissolution is expected Slope is proportional to dissolution rate Curvature of concentration profile indicates a phase transformation 20
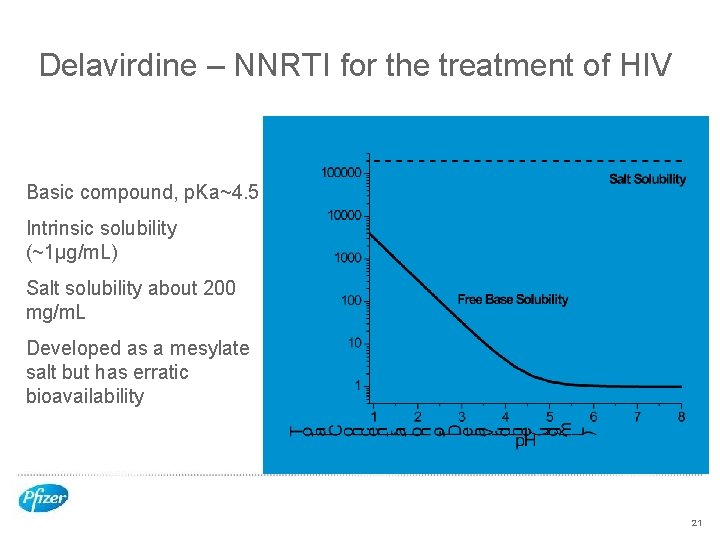
Delavirdine – NNRTI for the treatment of HIV Basic compound, p. Ka~4. 5 Intrinsic solubility (~1µg/m. L) Salt solubility about 200 mg/m. L Developed as a mesylate salt but has erratic bioavailability 21
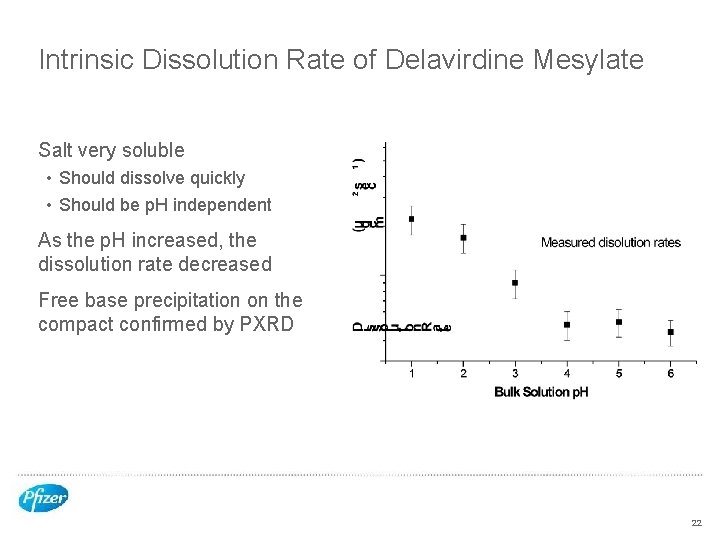
Intrinsic Dissolution Rate of Delavirdine Mesylate Salt very soluble • Should dissolve quickly • Should be p. H independent As the p. H increased, the dissolution rate decreased Free base precipitation on the compact confirmed by PXRD 22

What is happening? 23

Free base precipitation 24
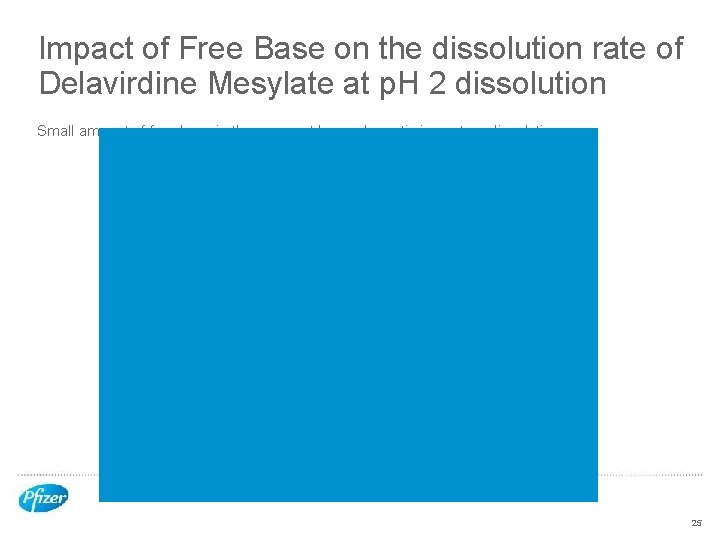
Impact of Free Base on the dissolution rate of Delavirdine Mesylate at p. H 2 dissolution Small amount of free base in the compact has a dramatic impact on dissolution 25
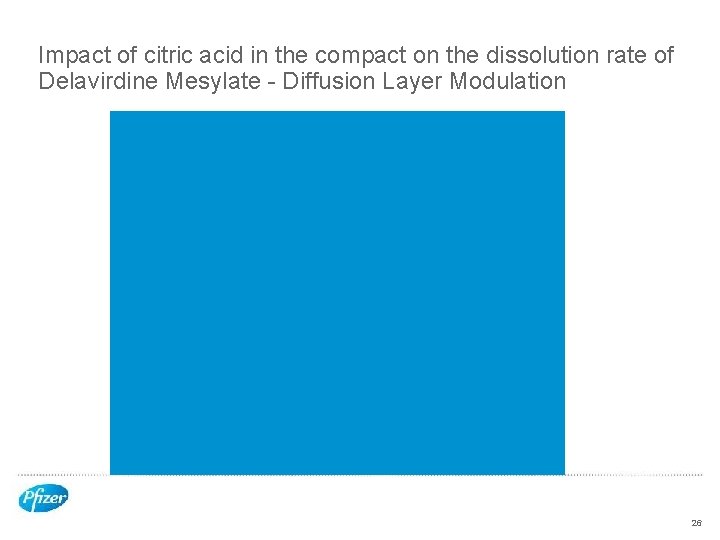
Impact of citric acid in the compact on the dissolution rate of Delavirdine Mesylate - Diffusion Layer Modulation 26
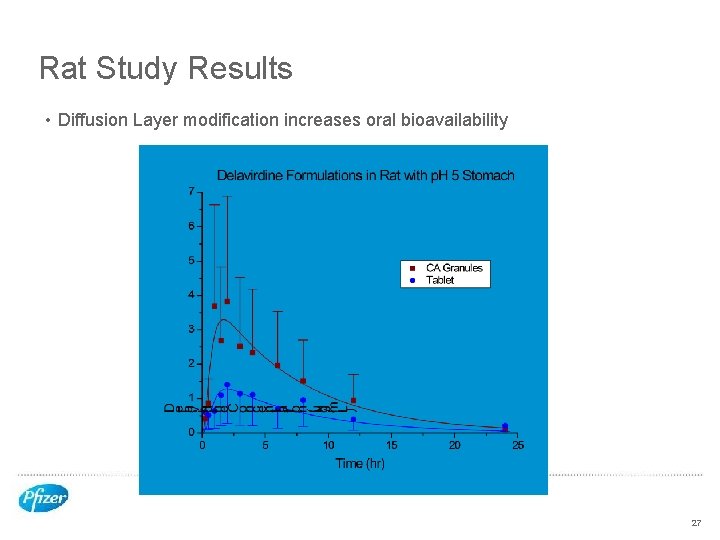
Rat Study Results • Diffusion Layer modification increases oral bioavailability 27
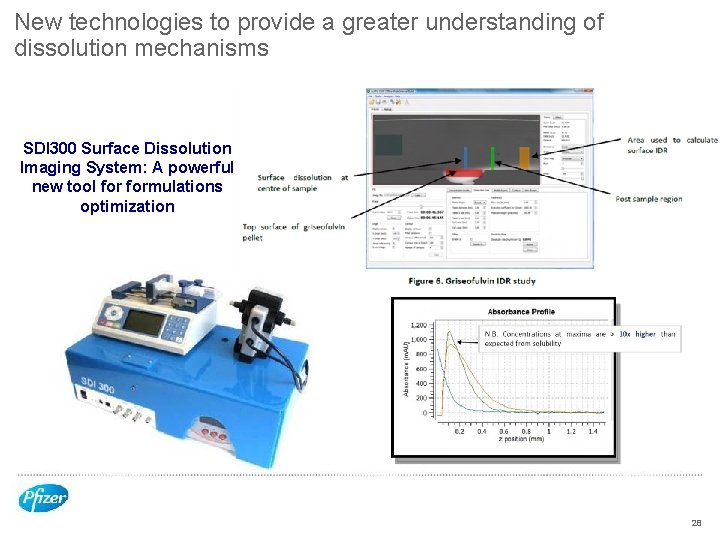
New technologies to provide a greater understanding of dissolution mechanisms SDI 300 Surface Dissolution Imaging System: A powerful new tool formulations optimization 28
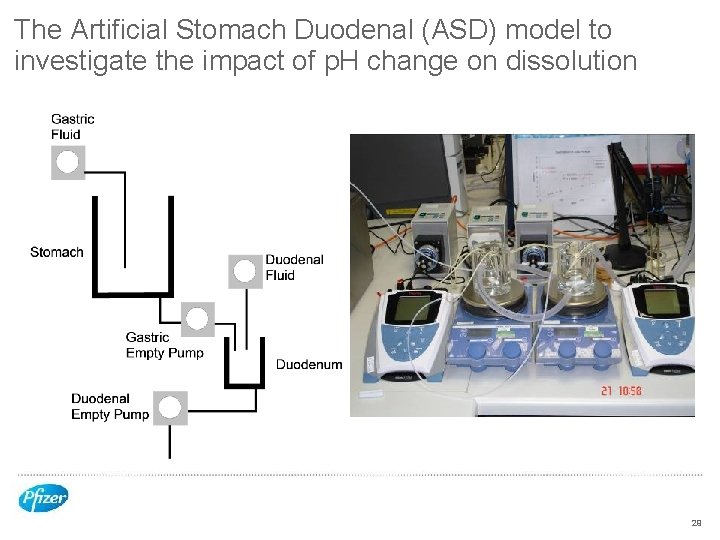
The Artificial Stomach Duodenal (ASD) model to investigate the impact of p. H change on dissolution 29
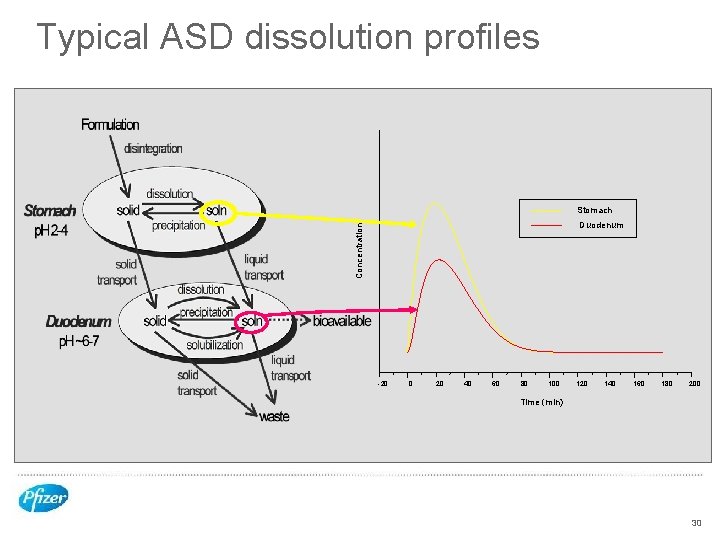
Typical ASD dissolution profiles Stomach Concentration Duodenum -20 0 20 40 60 80 100 120 140 160 180 200 Time (min) 30
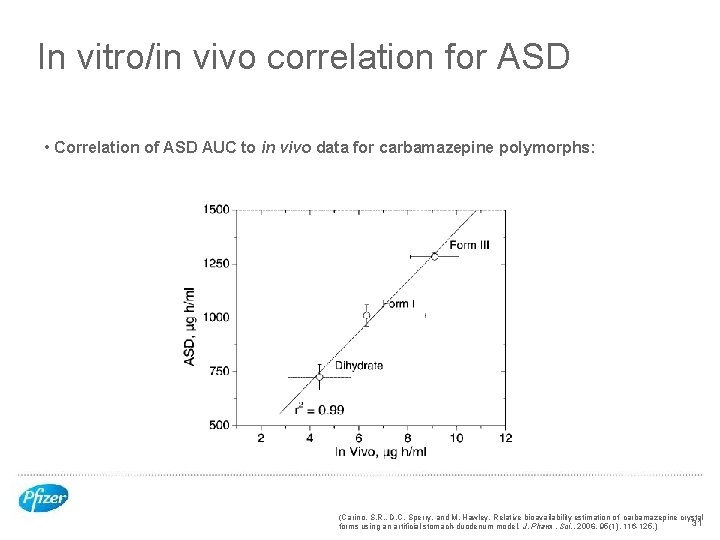
In vitro/in vivo correlation for ASD • Correlation of ASD AUC to in vivo data for carbamazepine polymorphs: (Carino, S. R. , D. C. Sperry, and M. Hawley, Relative bioavailability estimation of carbamazepine crystal 31 forms using an artificial stomach-duodenum model. J. Pharm. Sci. , 2006, 95(1), 116 -125. )

Formulations of compound X were being developed for rapid oral onset of action • Weak based with low solubility >p. H 4. 5 ~10 ug/m. L • High solubility at gastric p. H (>5 mg/m. L) • Three crystalline solid forms were available – Free base • Intrinsic solubility of 10 ug/m. L – Citrate salt • Intrinsic solubility of 20 mg/m. L – Mesylate salt • Intrinsic solubility of 80 mg/m. L 32

ASD data for Compound X solid forms with a p. H 4 stomach. Concentration in solution in the Stomach Concentration in solution in the Duodenum • The mesylate performed poorly in the duodenum with a gastric of p. H 4. 0 • Precipitation to free base? • The citrate salt performs the best in the duodenum compartment • Slower dissolution leading to less precipitation at higher p. H 33

p. H modulated dog model to monitor oral absorption of Compound X salts from stomachs of low and high p. H Pentagastrin treated dogs • Good precedent for use in dogs to reduce stomach p. H • 10 mcg/kg im Medtronic Bravo p. H telemetry systems – 15 minutes prior to dosing and 30 minutes post dosing Pantoprazole treated dogs • Low hepatic drug interaction potential and used in veterinary practise with dogs • 1 mg/kg iv 12 hours pre dose and 1 hour post dose Compound X formulated as rapidly disintegrating tablets of the free base, mesylate and citrate salts Tablets dose with the Bravo capsule on a fasted stomach with a small volume of water 34
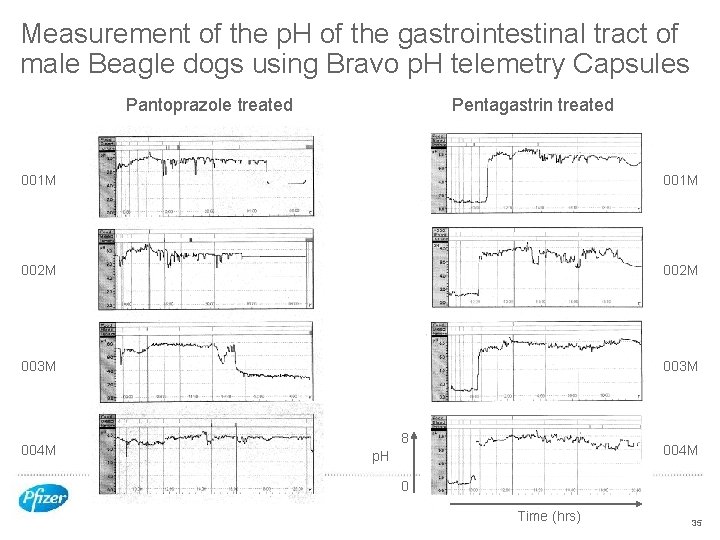
Measurement of the p. H of the gastrointestinal tract of male Beagle dogs using Bravo p. H telemetry Capsules Pantoprazole treated Pentagastrin treated 001 M 002 M 003 M 004 M 8 004 M p. H 0 Time (hrs) 35

Gastric p. H in male beagle dogs after the different pretreatments to control Gastric p. H Mean = 4. 6 + 2. 7 Mean = 6. 6 + 0. 7 Mean = 1. 3 + 0. 2 36
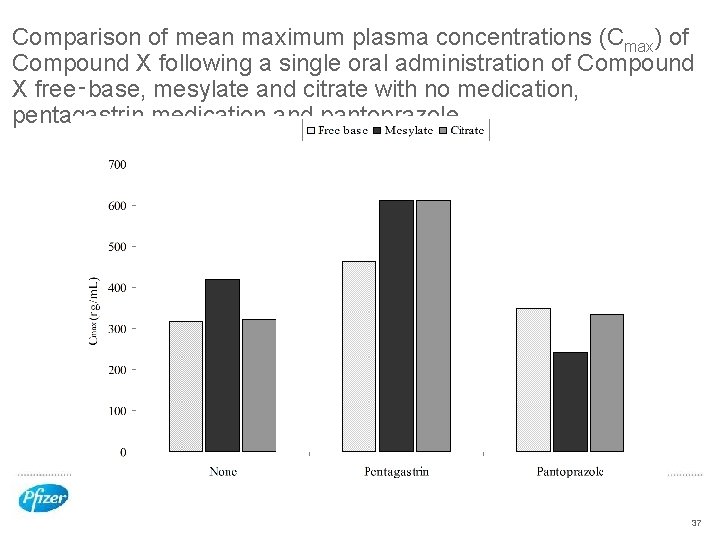
Comparison of mean maximum plasma concentrations (Cmax) of Compound X following a single oral administration of Compound X free‑base, mesylate and citrate with no medication, pentagastrin medication and pantoprazole 37
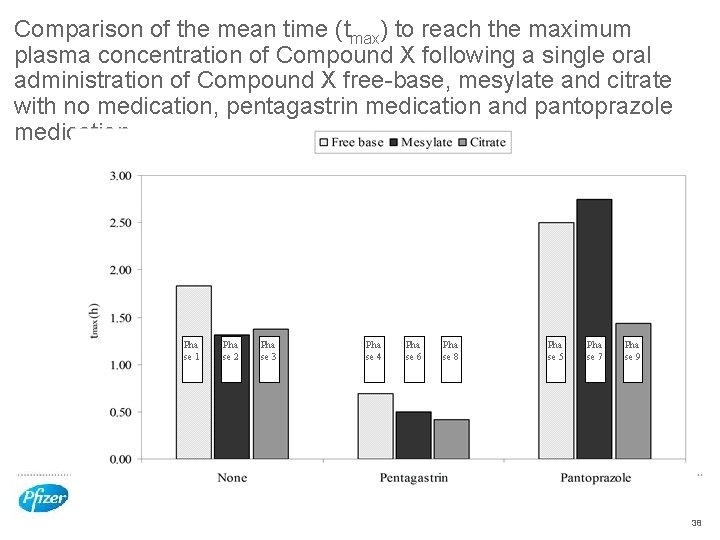
Comparison of the mean time (tmax) to reach the maximum plasma concentration of Compound X following a single oral administration of Compound X free-base, mesylate and citrate with no medication, pentagastrin medication and pantoprazole medication Pha se 1 Pha se 2 Pha se 3 Pha se 4 Pha se 6 Pha se 8 Pha se 5 Pha se 7 Pha se 9 38
p. H modulated dog model to monitor oral absorption of Compound X salts from stomachs of low and high p. H • All Compound X salts performed better at lower stomach p. H • Faster absorption as measured by shorter Tmax and Higher Cmax • Trend for the citrate salt to have more reliable performance across a wider p. H range • Supported by the ASD in vitro dissolution/precipitation model • Bravo p. H telemetry capsule worked successfully in this dog model • Drug pre-treatments successfully controlled dog stomach p. H to desired level • Fasted dog stomach highly variable 39
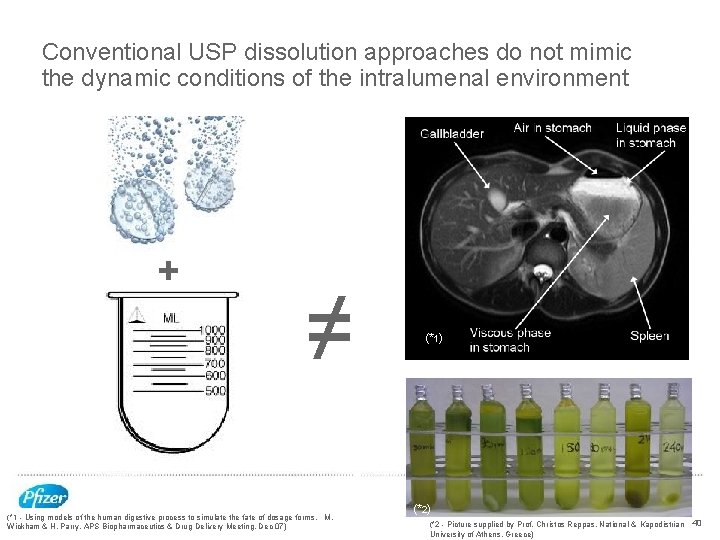
Conventional USP dissolution approaches do not mimic the dynamic conditions of the intralumenal environment + ≠ (*1 - Using models of the human digestive process to simulate the fate of dosage forms, M. Wickham & H. Parry, APS Biopharmaceutics & Drug Delivery Meeting, Dec 07) (*1) (*2 - Picture supplied by Prof. Christos Reppas, National & Kapodistrian 40 University of Athens, Greece)
New technologies now provide a view from the tablets perspective Daniel Bar-Shalom Faculty of Pharmaceutical Sciences University of Copenhagen 41
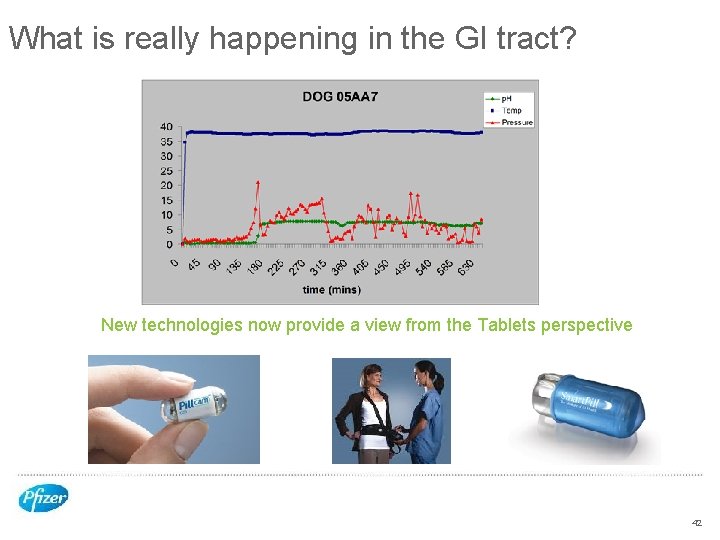
What is really happening in the GI tract? New technologies now provide a view from the Tablets perspective 42

Typical daily variation in gastric p. H in a health subject 43
Reduced acid secretion in the Stomach Hypochlorhydria and Achlorhydria Disease states know to be associated with reduced acid production • • • Malnutrition HIV/AIDs Gastric infections (incl H Pylori) Gastric inflammation and cancer Autoimmune diseases (pernicious anaemia, thyroid disorders) Genetic Disorders Surgical induced hypochlorhydria • Gastric resection, Vagotomy Drug induced hypochlorhydria • H 2 antagonists, Proton Pump Inhibitors, Antacids • Cannabis • Aspirin, alcohol Aged associated hypochlorhydria 44

Gastritis and Acid Secretion in the Elderly • Prevalence of gastric cancer and peptic ulcers more common and severe with advancing aging • Well established that gastric p. H in the elderly can be more variable and higher then young • 50% of all people over 65 have H pylori infection and with prevalence increasing with advancing age • 30% of all people over 60 have atrophic gastritis ‘Free-living Boston Elderly’ atrophic gastritis rates – 60 -60 – 70 -79 – >80 21% 37% • High Gastric p. H in the elderly • Quinine resin release study in 258 people over 65 s – 67% normal – 22% intermittent secretors – 11% had consistently p. H>3. 5 • p. H telemetry study in 79 people over 65 – 11% had p. H consistently p. H>5. 0 – Equated to ~5 M Americans in 2020 • Achlorhydria in the elderly associated with poor absorption of nutrients – Ca, Fe, folic acid, Vit B 6 and B 12 45
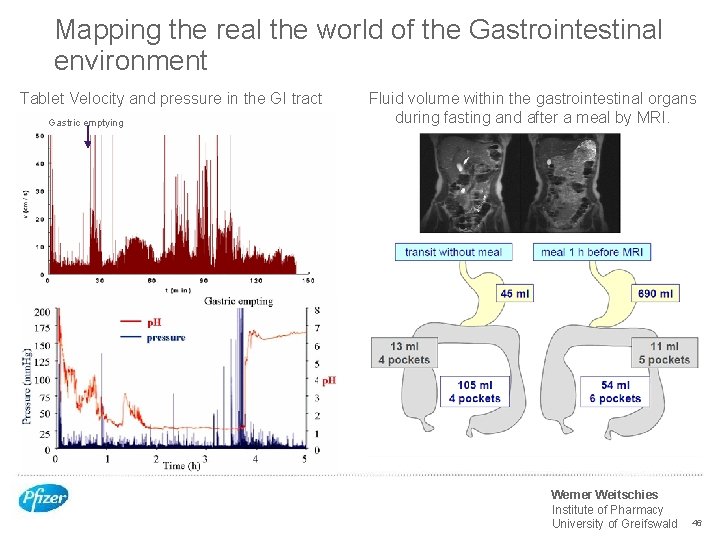
Mapping the real the world of the Gastrointestinal environment Tablet Velocity and pressure in the GI tract Gastric emptying Fluid volume within the gastrointestinal organs during fasting and after a meal by MRI. Werner Weitschies Institute of Pharmacy University of Greifswald 46

What should a biorelevant dissolution system consider? • Changing p. H, digestive enzyme and bile levels • Removal of dissolved drugs from the intestinal lumen • Discontinuity of movement of the dosage forms → Velocities of dosage forms up to 50 cm/s • Simulation of pressure waves of physiological power → Pressure values up to 300 mbar (~230 mm Hg) • Interrupted contact of the dosage form to the medium • Device should be able to operate with small fluid volumes 47

Dynamic Gastric Model Fully automated, computer controlled dynamic model of human stomach System modelled closely on the human stomach Can process real food and drugs in real time Includes stomach volume, peristaltic motion and continuous gastric secretions, simulating physical and biochemical processes Has been used for some time in food research Application to pharmaceutical products more recent. Can be applied to a variety of the dissolution challenges. 48

TNO TIM-1 dynamic dissolution model Stomach and small intestinal model which mimics key aspects of intestinal physiology including: • GI p. H profiles and transit times • Secretion of gastric acid and enzymes (pepsin, lipase) • Secretion of bile, pancreatic juice • Absorption of digested products via dialysis Provides information on release kinetics in the intestinal lumen and availability for absorption (bioaccessibility) Fed/fasted studies completed with micronized tablet, SDD tablet and nanomilled suspension 49
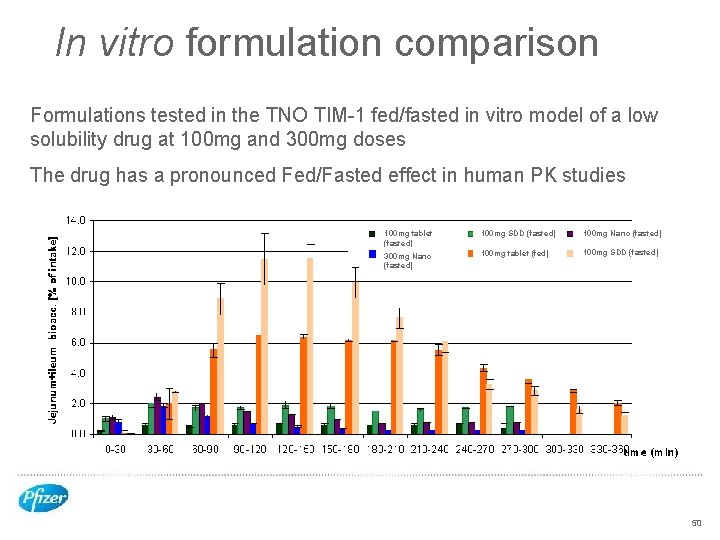
In vitro formulation comparison Formulations tested in the TNO TIM-1 fed/fasted in vitro model of a low solubility drug at 100 mg and 300 mg doses The drug has a pronounced Fed/Fasted effect in human PK studies 100 mg tablet (fasted) 100 mg SDD (fasted) 100 mg Nano (fasted) 300 mg Nano (fasted) 100 mg tablet (fed) 100 mg SDD (fasted) 50
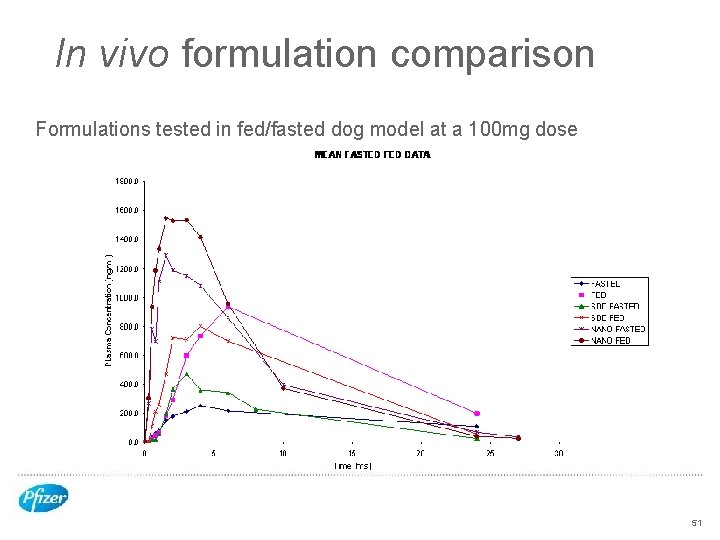
In vivo formulation comparison Formulations tested in fed/fasted dog model at a 100 mg dose 51

TNO TIM-1 summary • Strong positive food effect seen in the clinic with high fat meals replicated in the TNO TIM-1 model • Recovery results formulations tested typically >75% • Formulation ranking – TNO TIM-1 • SDD > nanomilled suspension > micronised tablet – In vivo dog model • Nano Milled > SDD > micronised tablet – In addition, both demonstrated reduced variability • Still need to prove relevance to clinical performance in humans 52

University of Greifswald – Dissolution Stress Tester Developed specifically to mimic GI specific pressure events, GI motility and intermittent contact of dosage form with water. Garbacz et al. Eur J Pharm Biopharm (2008) 70: 421 -428 Werner Weitschies Institute of Pharmacy University of Greifswald 53
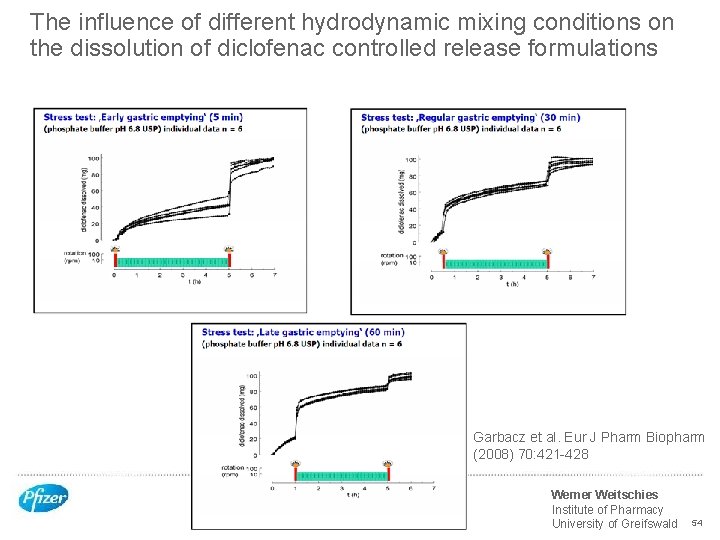
The influence of different hydrodynamic mixing conditions on the dissolution of diclofenac controlled release formulations Garbacz et al. Eur J Pharm Biopharm (2008) 70: 421 -428 Werner Weitschies Institute of Pharmacy University of Greifswald 54

Summary Dissolution testing has served us well over the years Provide the quality controlled required to help ensure patients get the full benefits of the medicines we develop Catalysed the debate leading to SUPAC, IVIVC, BCS, Qb. D solutions Greater scientific understanding is going to be required moving forward More tricky compounds and formulations coming through Enable robust formulation development and commercialisation Still struggling to predict clinical outcomes p. H dependant and low solubility compounds Integrate dissolution data into predictive PK packages Robustness of some controlled release technologies We are now developing a much better understanding of the GI environment Better dissolution tools in development Going to be tough validating with new compounds and formulations 55

Many thanks to Pfizer Sandwich Mark Mc. Allister, Mei Wong, Kiyo Sugano, Kelly Jones Pfizer Groton Michael Hawley, Rong Li, Kazuko Sagawa 56
- Slides: 56