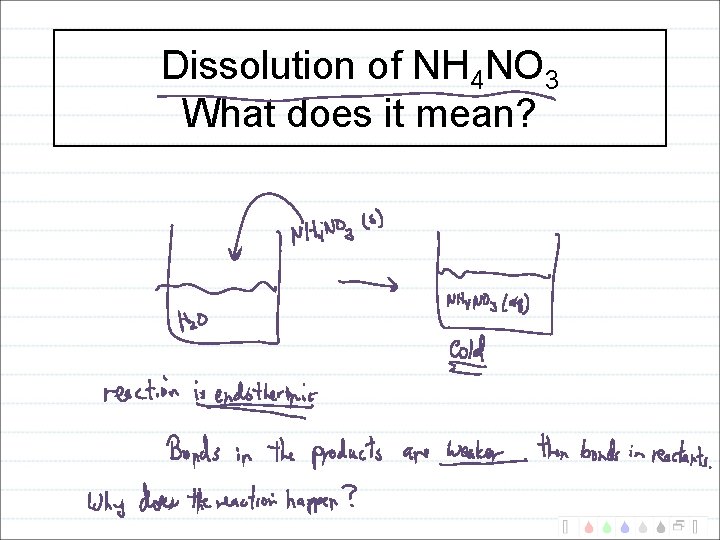
Dissolution of NH 4 NO 3 What does









- Slides: 55

Dissolution of NH 4 NO 3 What does it mean?

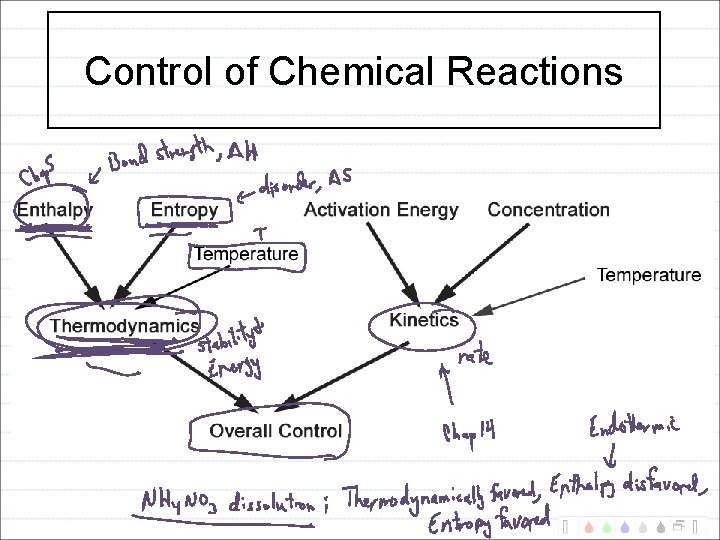
Control of Chemical Reactions

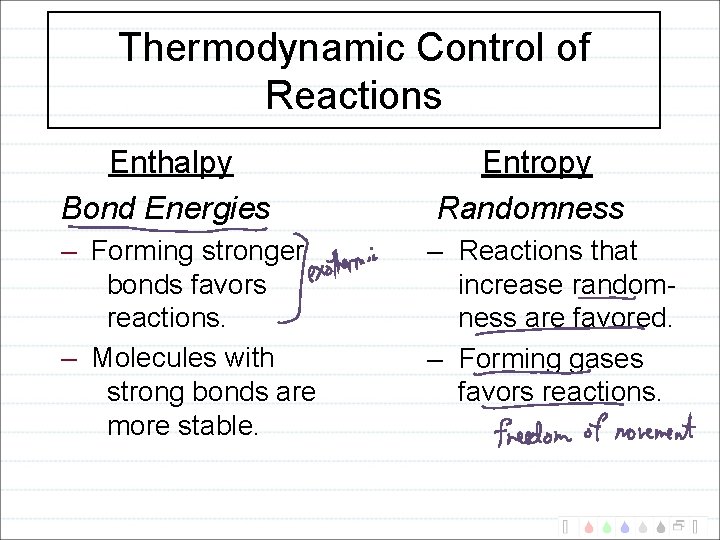
Thermodynamic Control of Reactions Enthalpy Bond Energies Entropy Randomness – Forming stronger bonds favors reactions. – Molecules with strong bonds are more stable. – Reactions that increase randomness are favored. – Forming gases favors reactions.

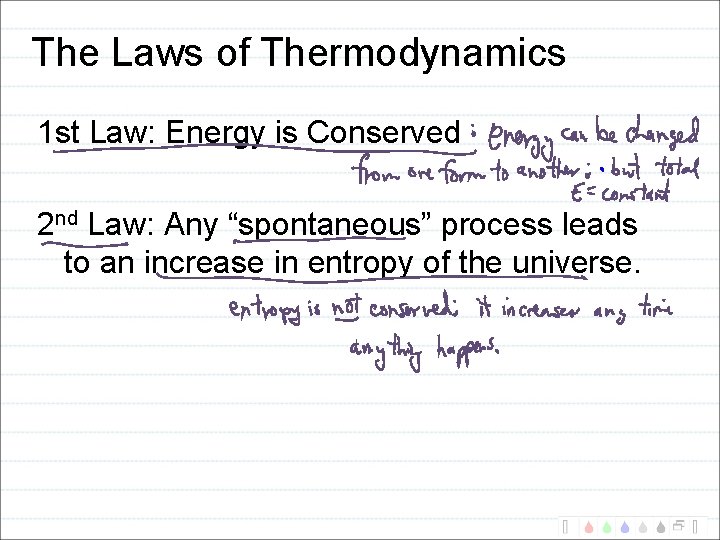
The Laws of Thermodynamics 1 st Law: Energy is Conserved 2 nd Law: Any “spontaneous” process leads to an increase in entropy of the universe.

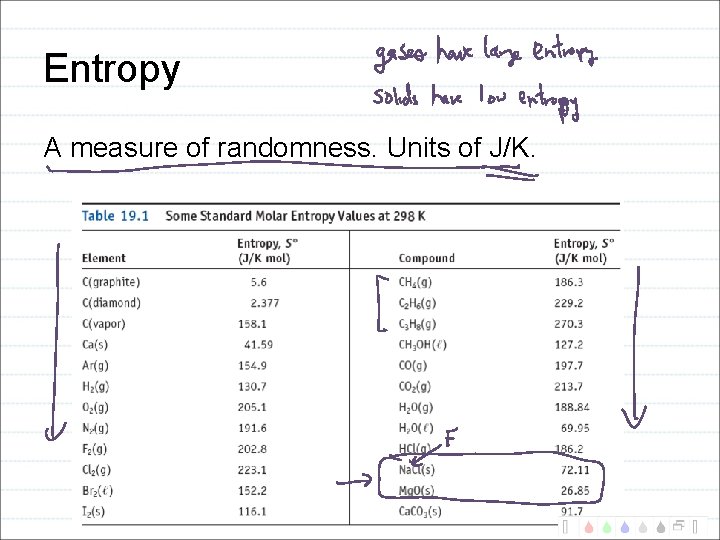
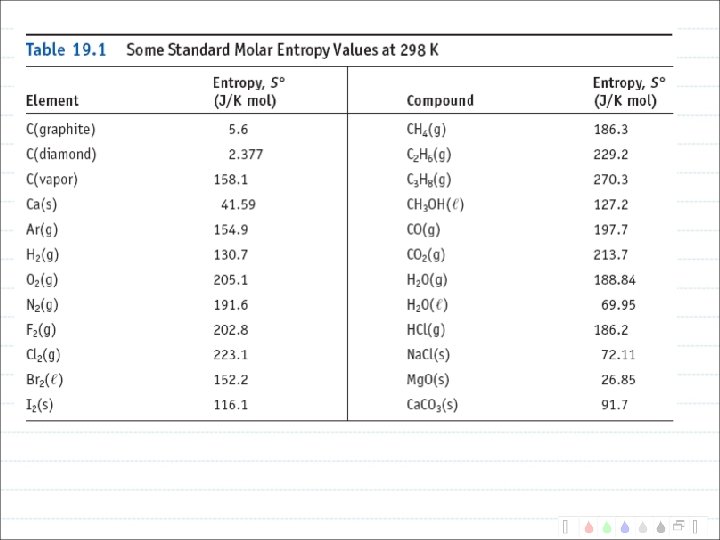
Entropy A measure of randomness. Units of J/K.

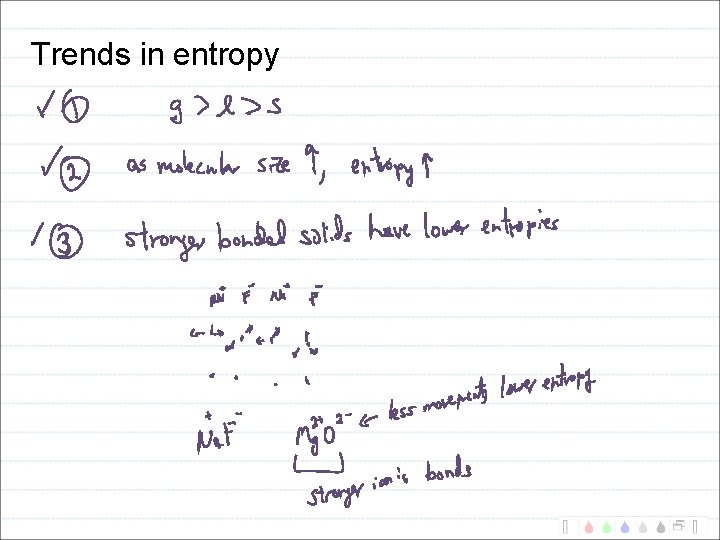
Trends in entropy

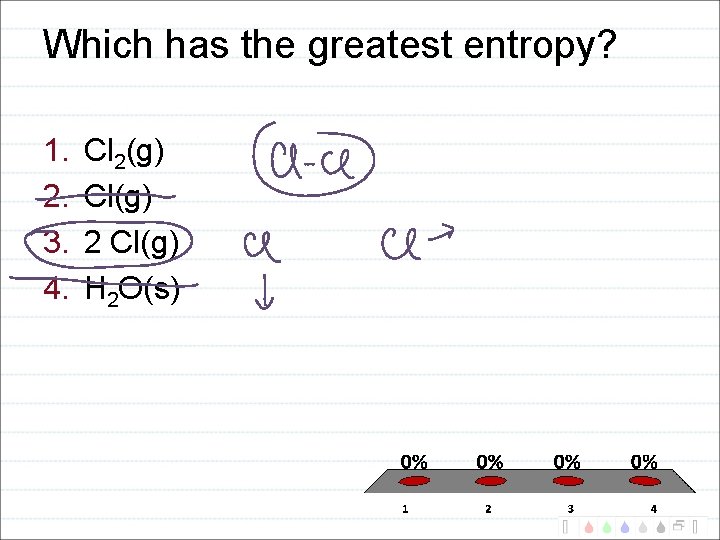
Which has the greatest entropy? 1. 2. 3. 4. Cl 2(g) Cl(g) 2 Cl(g) H 2 O(s)

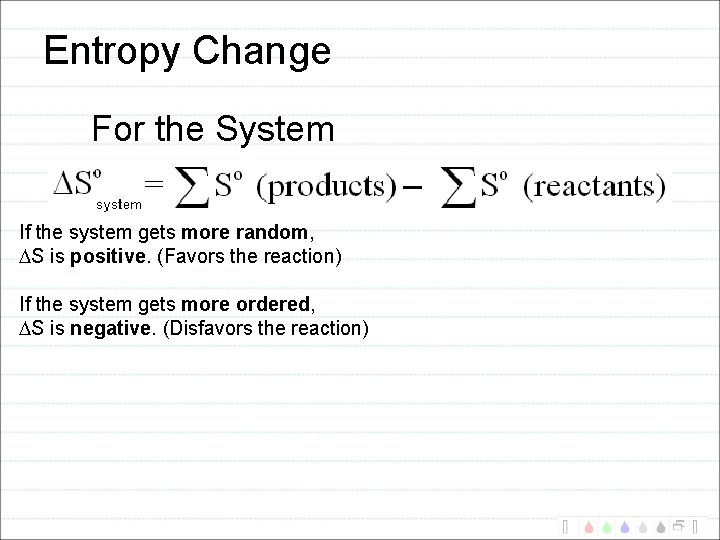
Entropy Change For the System If the system gets more random, S is positive. (Favors the reaction) If the system gets more ordered, S is negative. (Disfavors the reaction)

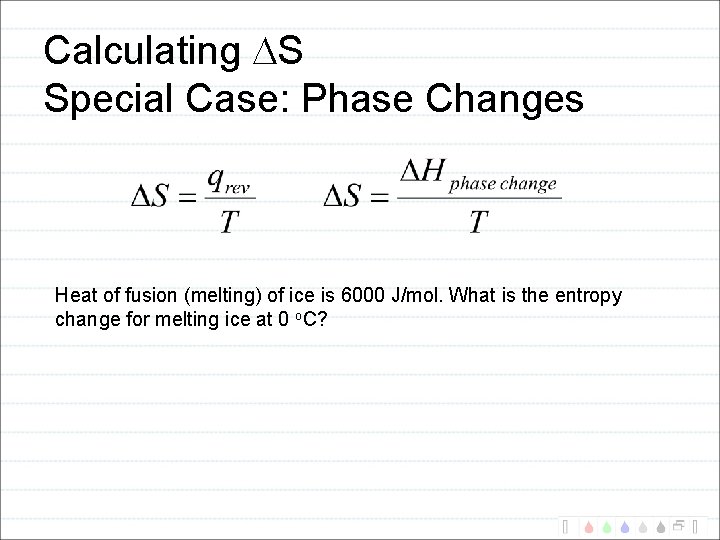
Calculating S Special Case: Phase Changes Heat of fusion (melting) of ice is 6000 J/mol. What is the entropy change for melting ice at 0 o. C?

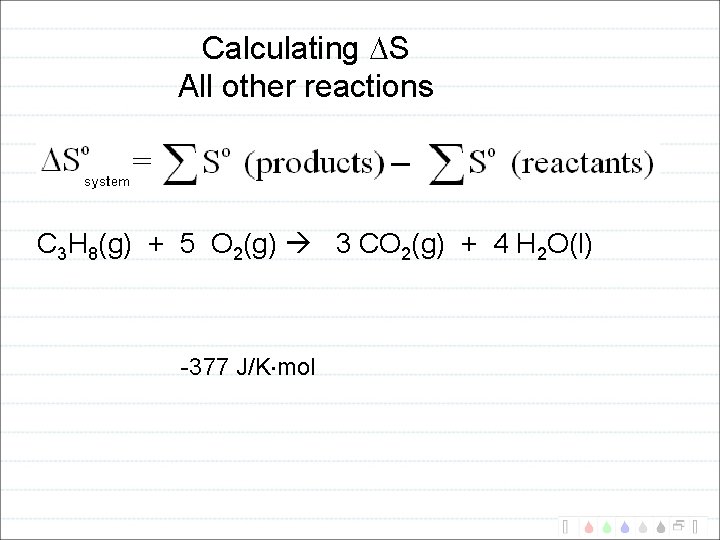
Calculating S All other reactions C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) -377 J/K mol

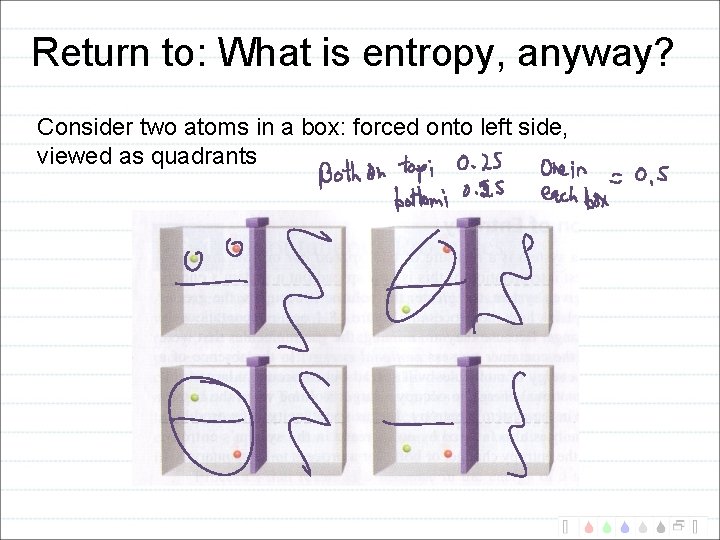
Return to: What is entropy, anyway? Consider two atoms in a box: forced onto left side, viewed as quadrants

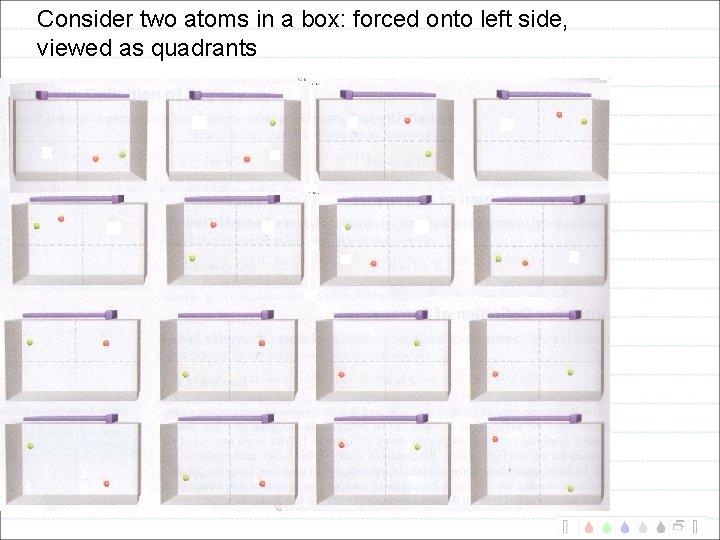
Consider two atoms in a box: forced onto left side, viewed as quadrants


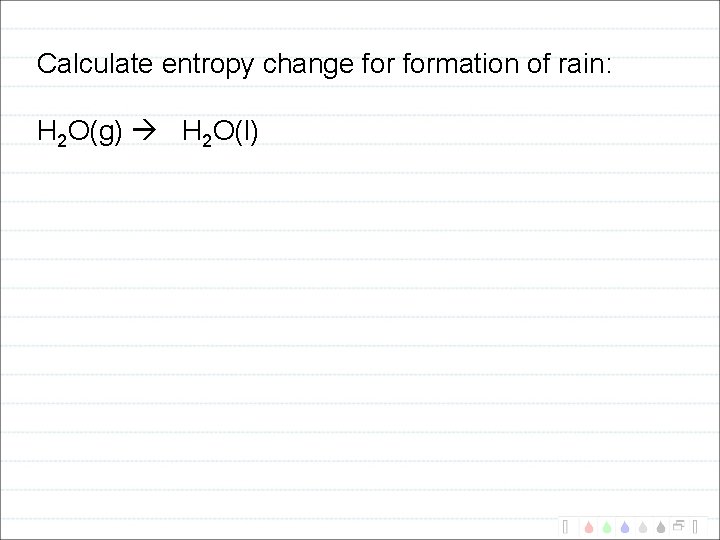
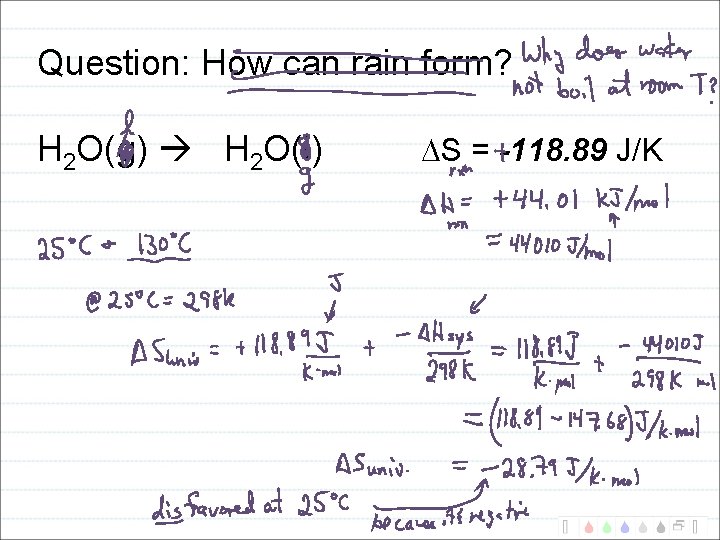
Calculate entropy change formation of rain: H 2 O(g) H 2 O(l)

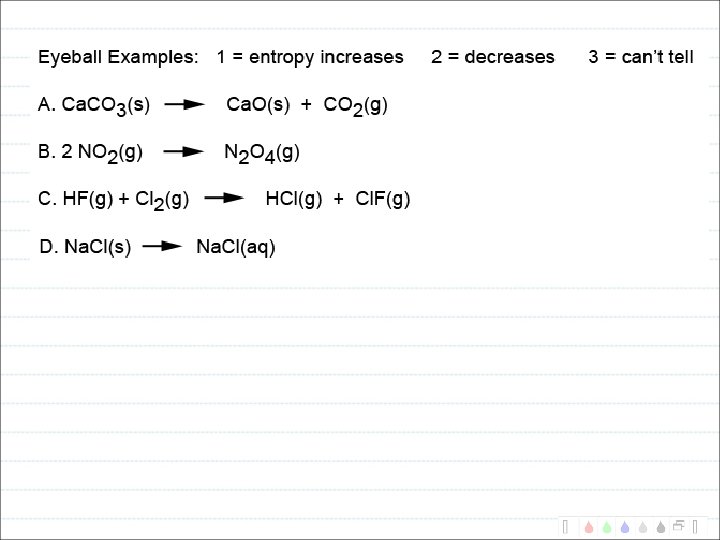
What types of reactions lead to increased entropy?


1 1. Inc 2. Dec 3. Can’t tell

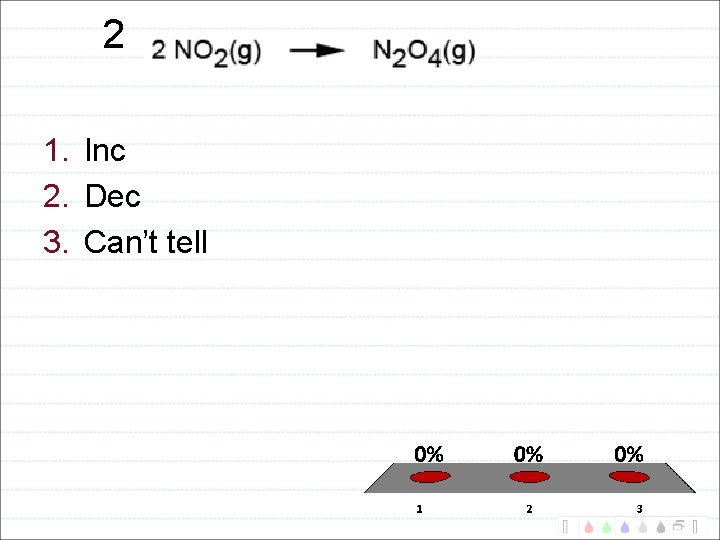
2 1. Inc 2. Dec 3. Can’t tell

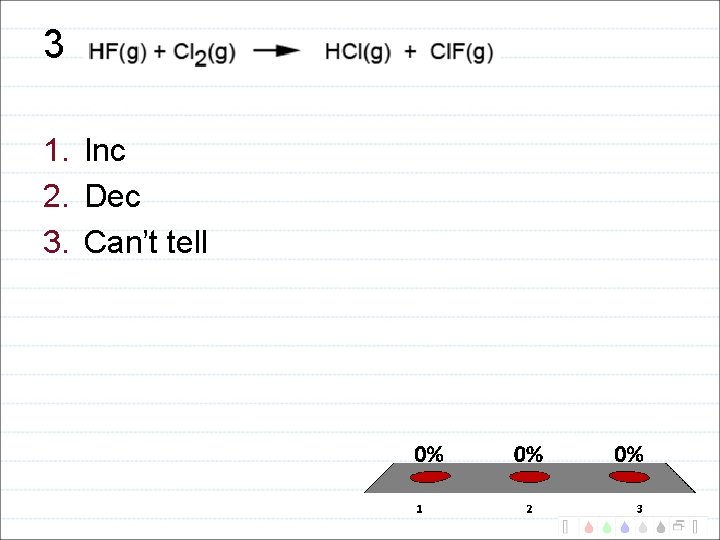
3 1. Inc 2. Dec 3. Can’t tell

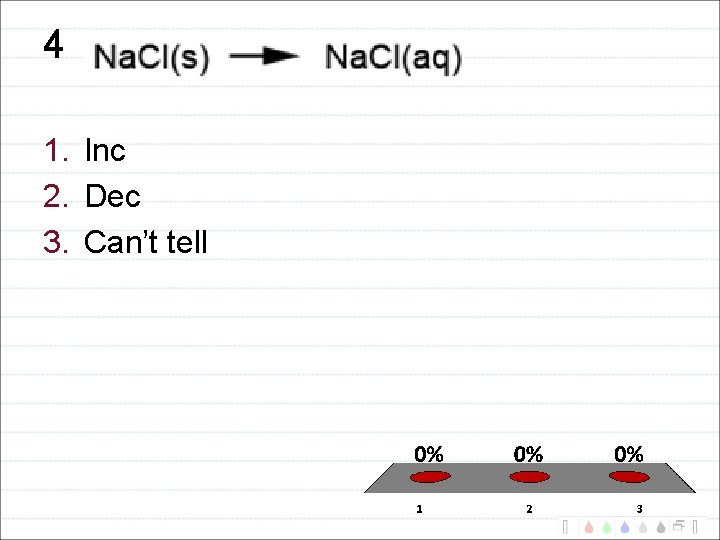
4 1. Inc 2. Dec 3. Can’t tell

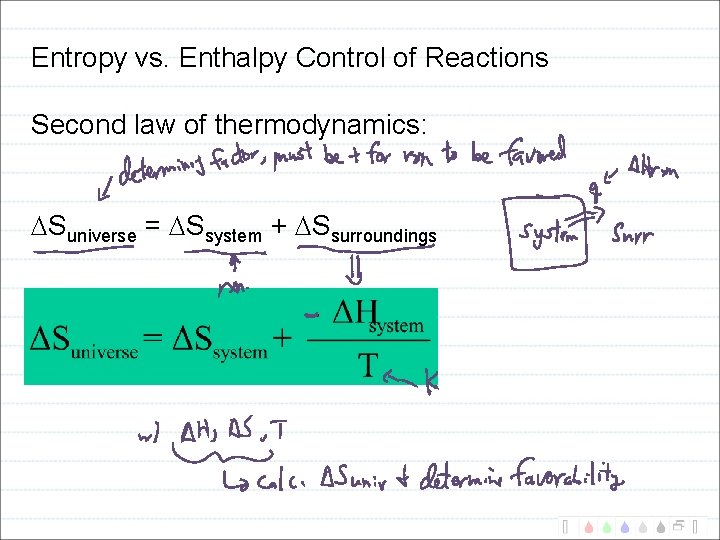
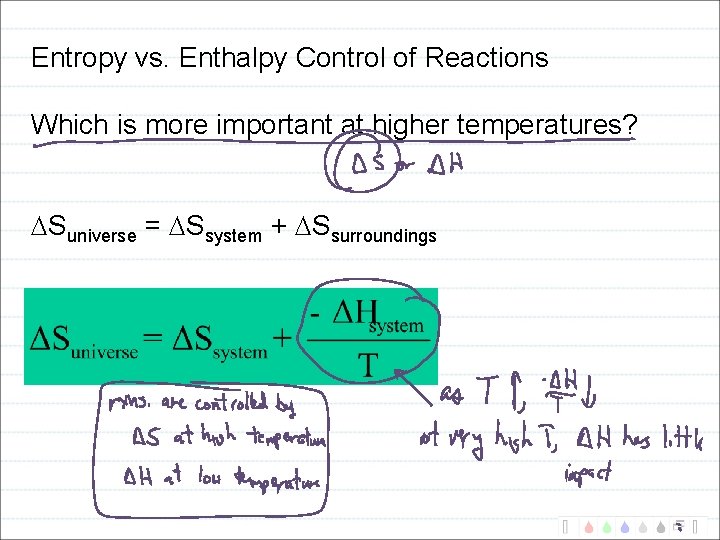
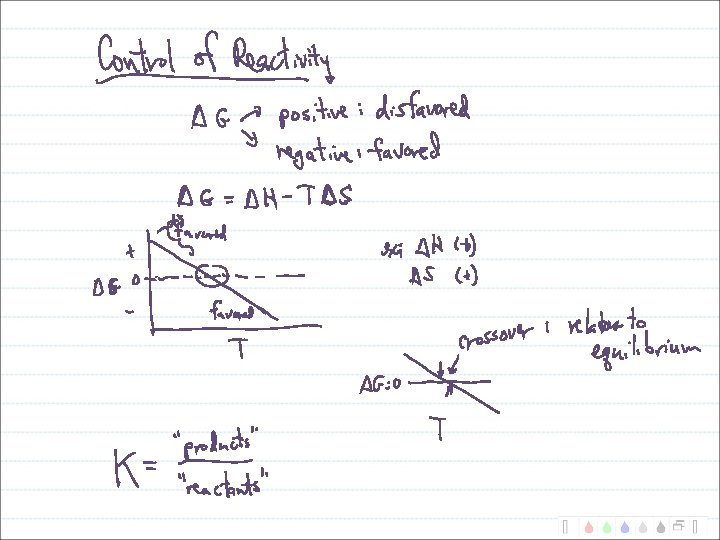
Entropy vs. Enthalpy Control of Reactions Second law of thermodynamics: Suniverse = Ssystem + Ssurroundings

Question: How can rain form? H 2 O(g) H 2 O(l) S = -118. 89 J/K

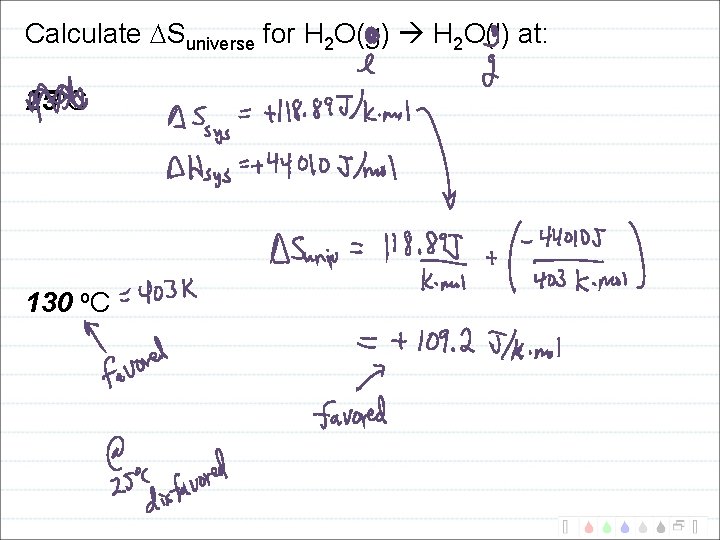
Calculate Suniverse for H 2 O(g) H 2 O(l) at: 25 o. C 130 o. C

Entropy vs. Enthalpy Control of Reactions Which is more important at higher temperatures? Suniverse = Ssystem + Ssurroundings

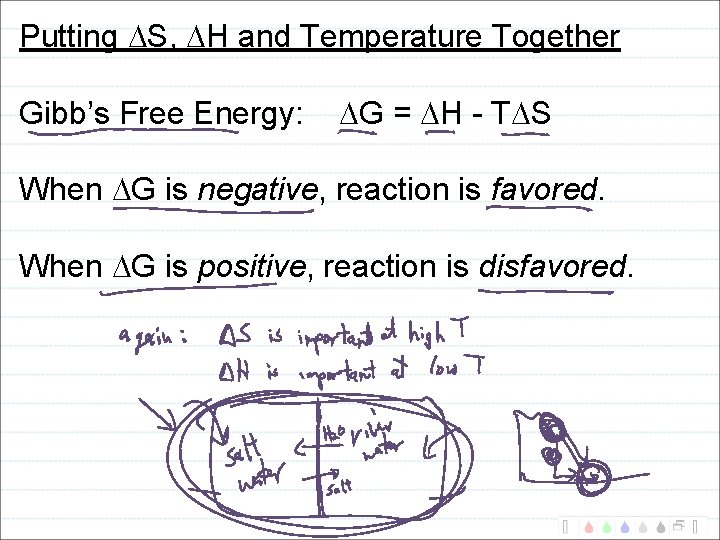
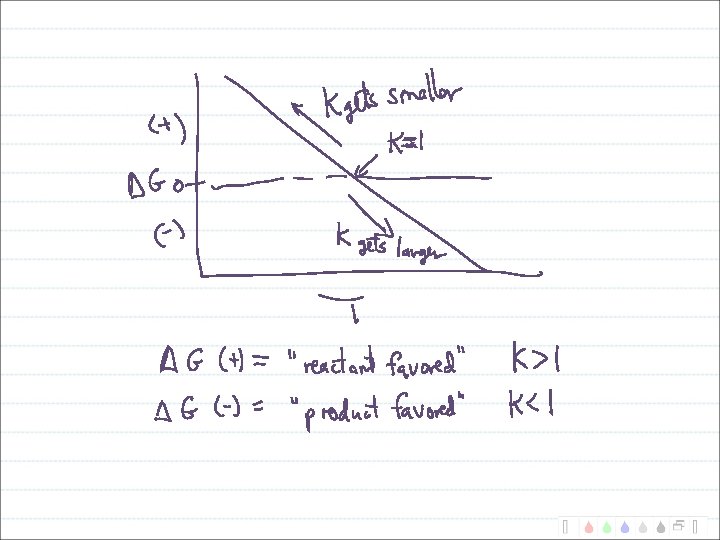
Putting S, H and Temperature Together Gibb’s Free Energy: G = H - T S When G is negative, reaction is favored. When G is positive, reaction is disfavored.

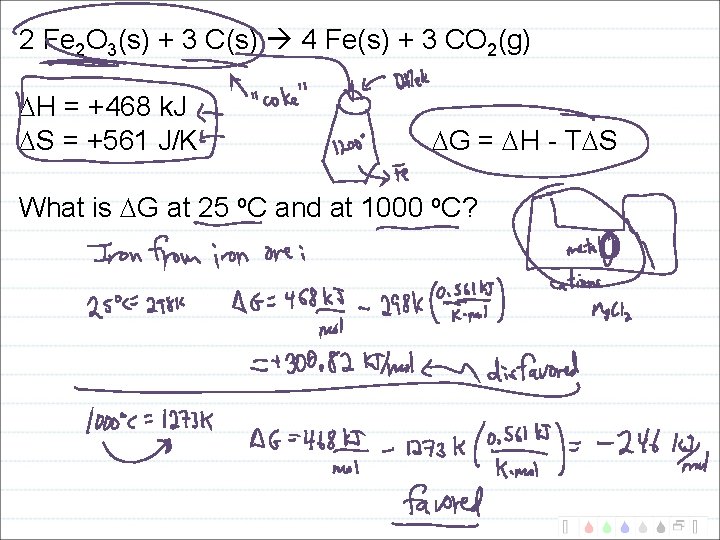
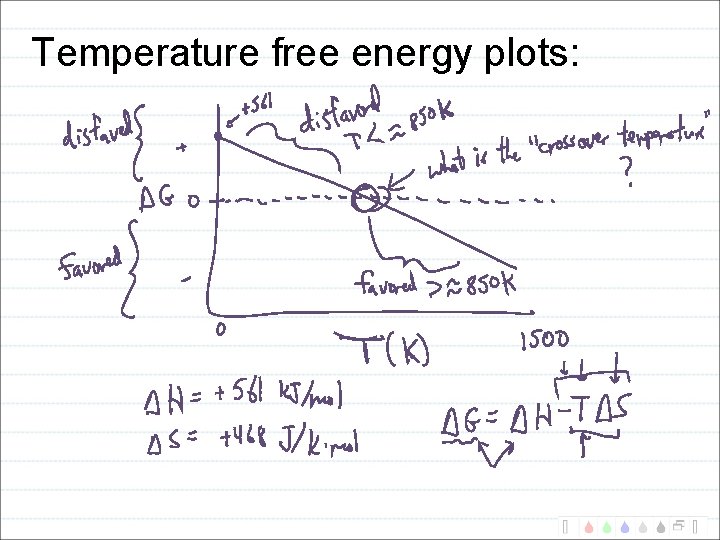
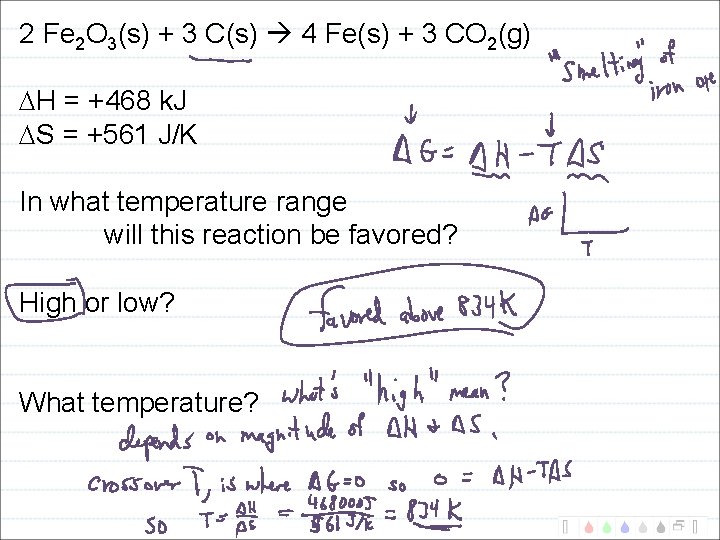
2 Fe 2 O 3(s) + 3 C(s) 4 Fe(s) + 3 CO 2(g) H = +468 k. J S = +561 J/K G = H - T S What is G at 25 o. C and at 1000 o. C?

Breaking bonds releases energy: 1. True 2. False

Bond energies and enthalpy change review:

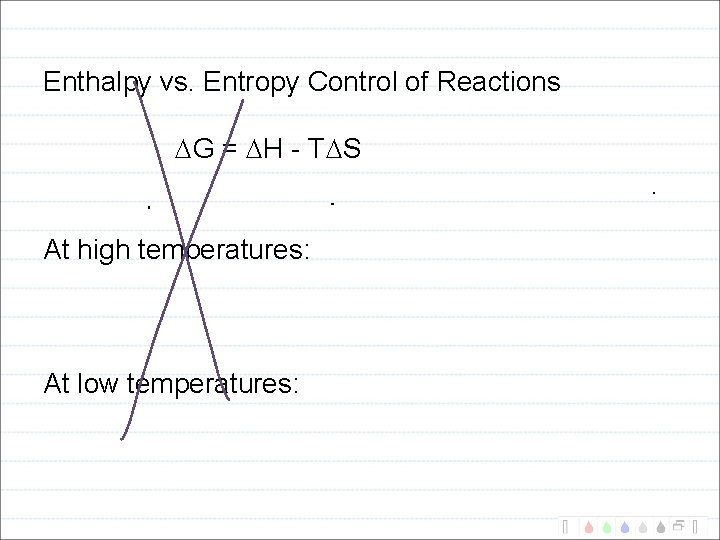
Enthalpy vs. Entropy Control of Reactions G = H - T S At high temperatures: At low temperatures:

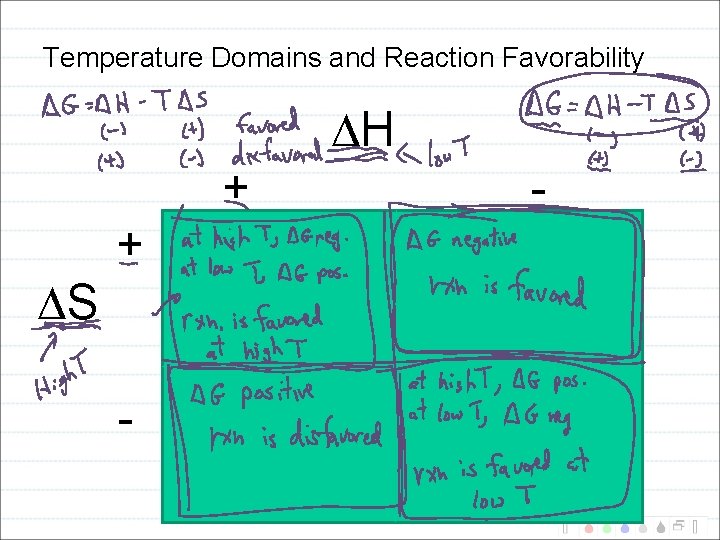
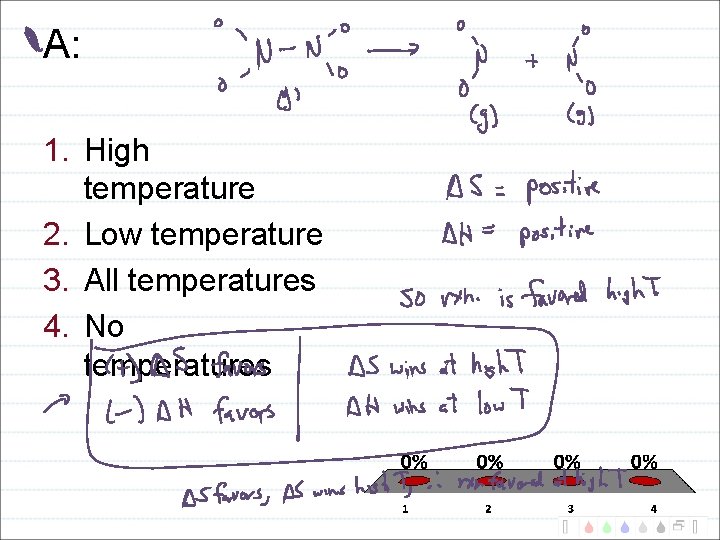
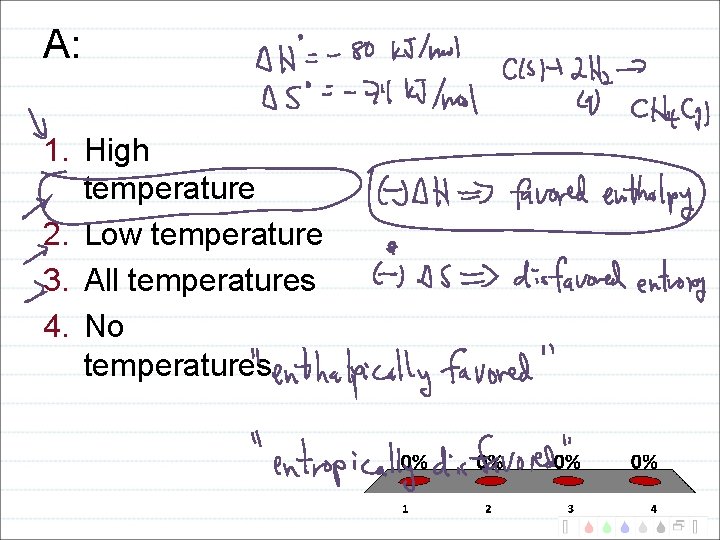
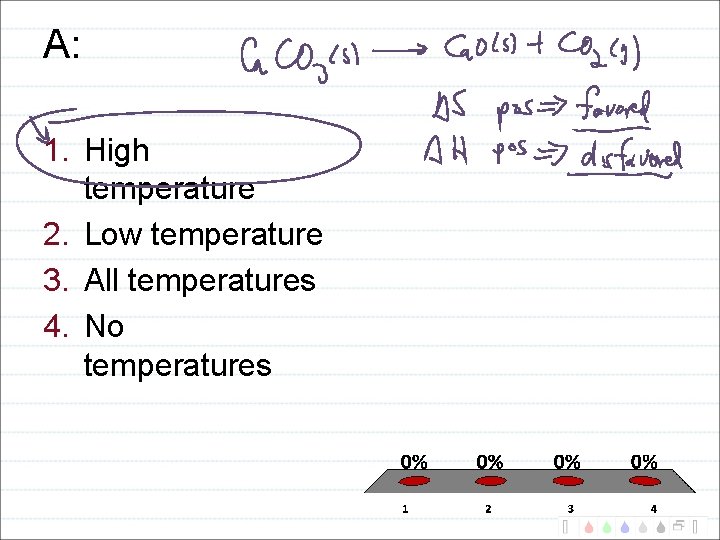
Temperature Domains and Reaction Favorability H + S + - -

A: 1. High temperature 2. Low temperature 3. All temperatures 4. No temperatures

A: 1. High temperature 2. Low temperature 3. All temperatures 4. No temperatures

A: 1. High temperature 2. Low temperature 3. All temperatures 4. No temperatures

A: 1. High temperature 2. Low temperature 3. All temperatures 4. No temperatures

Temperature free energy plots:

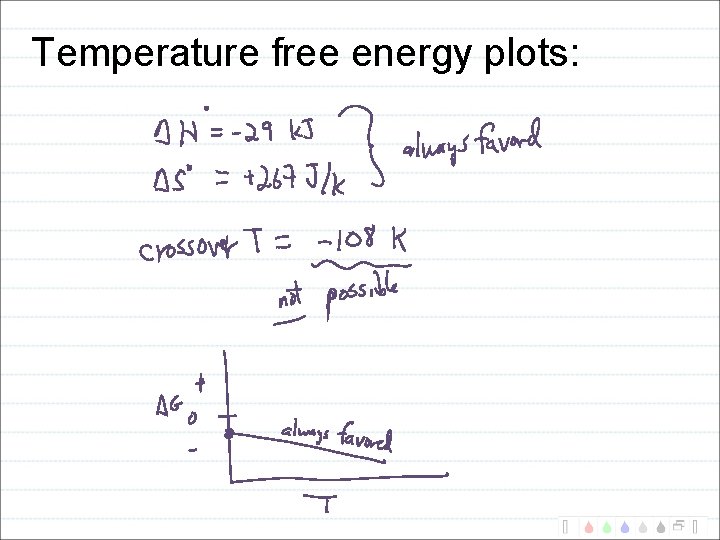
Temperature free energy plots:

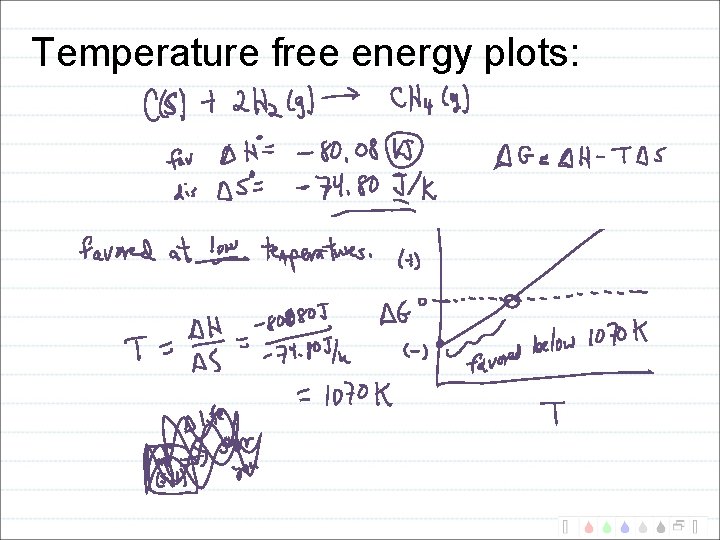
Temperature free energy plots:

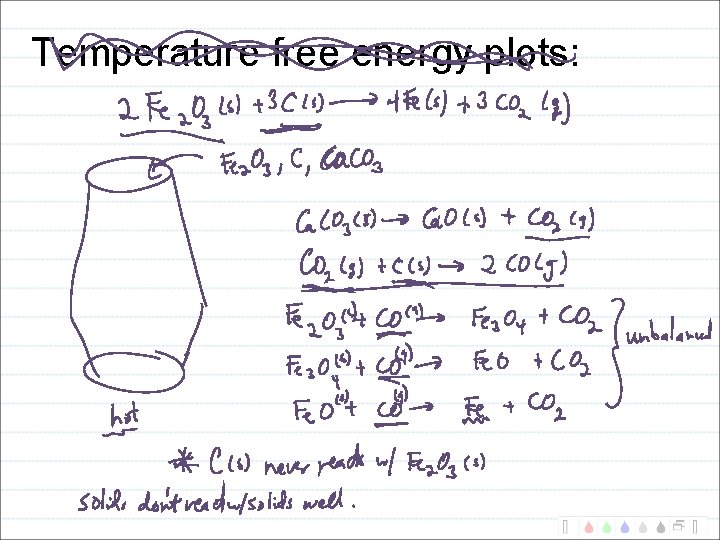
Temperature free energy plots:

2 Fe 2 O 3(s) + 3 C(s) 4 Fe(s) + 3 CO 2(g) H = +468 k. J S = +561 J/K In what temperature range will this reaction be favored? High or low? What temperature?














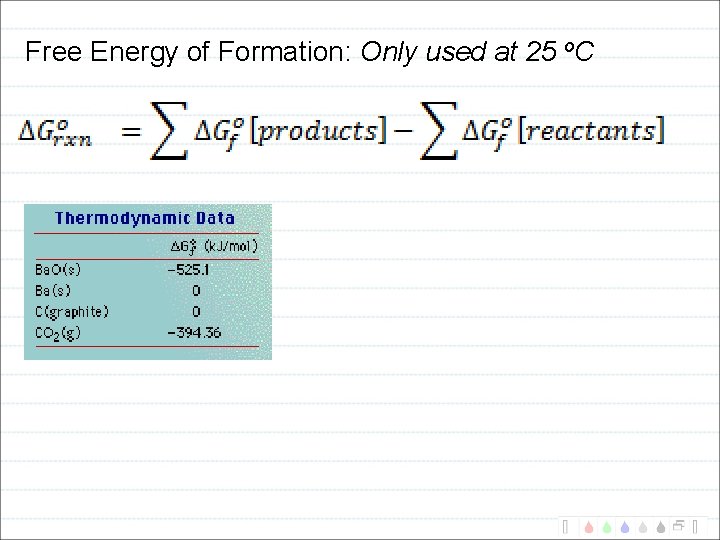
Free Energy of Formation: Only used at 25 o. C

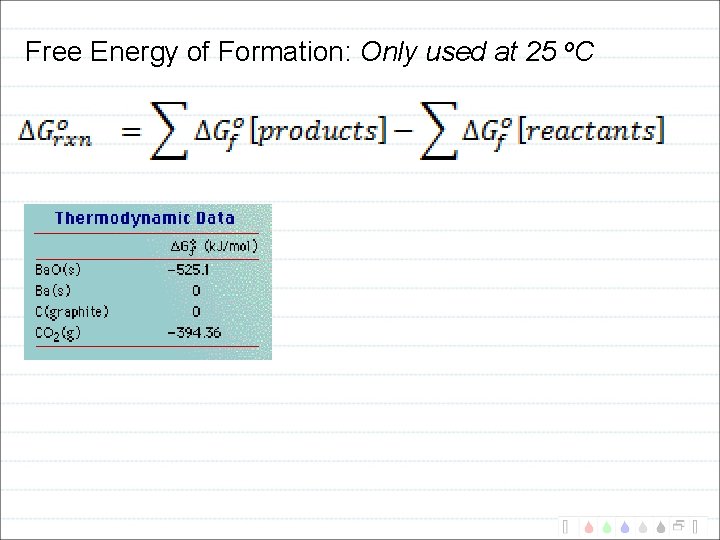
Free Energy of Formation: Only used at 25 o. C
