Dissolution and Solubility Processes Dissolutionprecipitation equilibria affect many
Dissolution and Solubility Processes • • • Dissolution-precipitation equilibria affect many soil processes, plant growth, etc Dissolution is the disintegration or dissolving of a mineral or compound into solution Dissolution occurs when the soil solution is undersaturated. "Most soil solutions are undersaturated with respect to the inorganic mineral components in the soil, which means that these soils are in a continuous state of dissolution. "
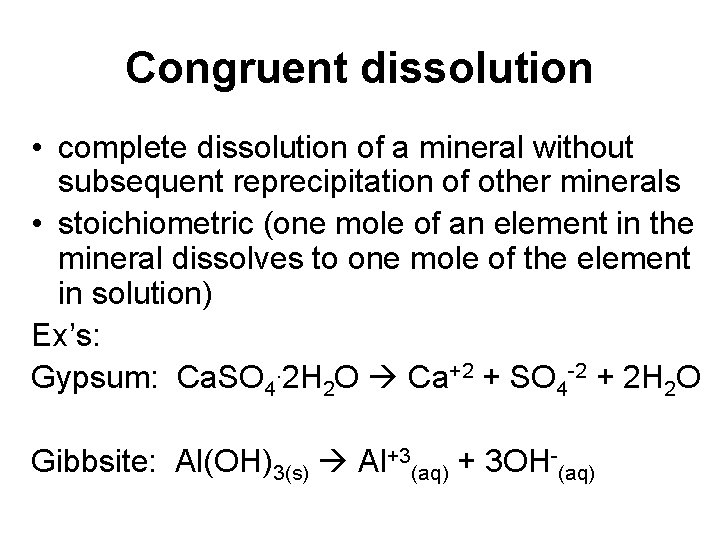
Congruent dissolution • complete dissolution of a mineral without subsequent reprecipitation of other minerals • stoichiometric (one mole of an element in the mineral dissolves to one mole of the element in solution) Ex’s: Gypsum: Ca. SO 4. 2 H 2 O Ca+2 + SO 4 -2 + 2 H 2 O Gibbsite: Al(OH)3(s) Al+3(aq) + 3 OH-(aq)
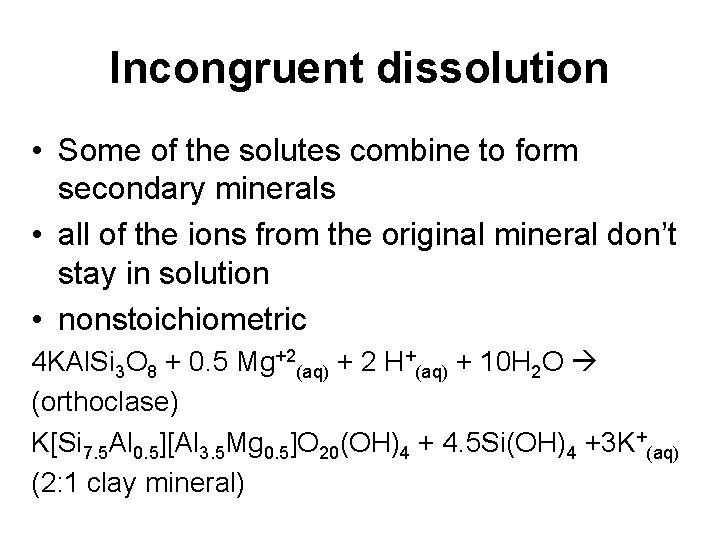
Incongruent dissolution • Some of the solutes combine to form secondary minerals • all of the ions from the original mineral don’t stay in solution • nonstoichiometric 4 KAl. Si 3 O 8 + 0. 5 Mg+2(aq) + 2 H+(aq) + 10 H 2 O (orthoclase) K[Si 7. 5 Al 0. 5][Al 3. 5 Mg 0. 5]O 20(OH)4 + 4. 5 Si(OH)4 +3 K+(aq) (2: 1 clay mineral)
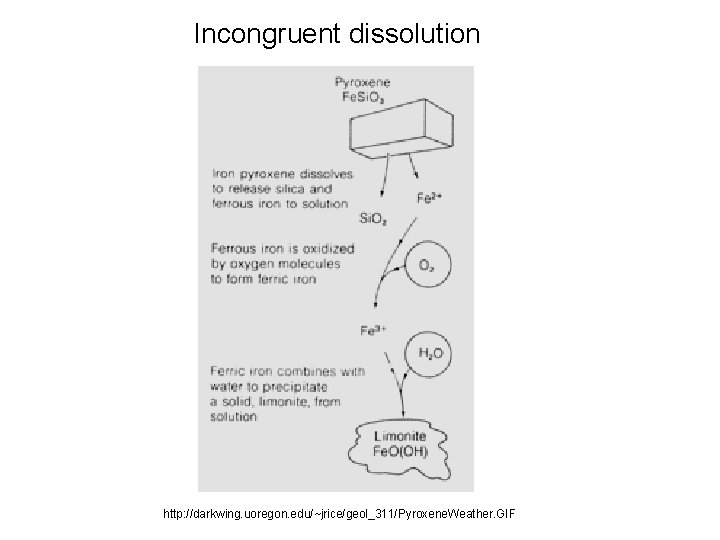
Incongruent dissolution http: //darkwing. uoregon. edu/~jrice/geol_311/Pyroxene. Weather. GIF
Silicate weathering characteristics • • Loss of tetrahedrally coordinated Al Oxidation of Fe(II) to Fe(III) Consumption of protons (H+) Release of silica and the metal cations Na+, K+, Mg+2, and Ca+2 Ex: feldspars + CO 2 + H 2 O clay minerals + ions + bicarbonate
Dissolution in nature Water, CO 2, and carbonic acid (CO 2 + H 2 O H 2 CO 3 H+ + HCO 3 -) dissolve rocks and soil minerals and release ions to soil solution (nutrients, salts, toxins, metals, etc. ) Ex: feldspars + CO 2 + H 2 O clay minerals + ions
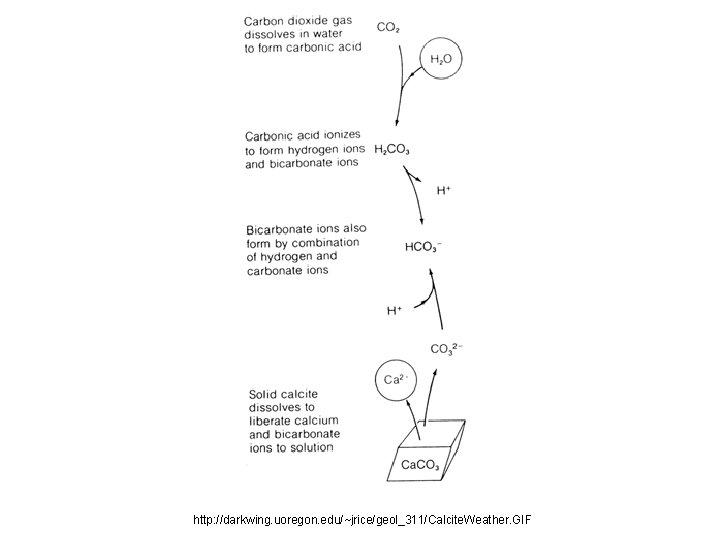
http: //darkwing. uoregon. edu/~jrice/geol_311/Calcite. Weather. GIF
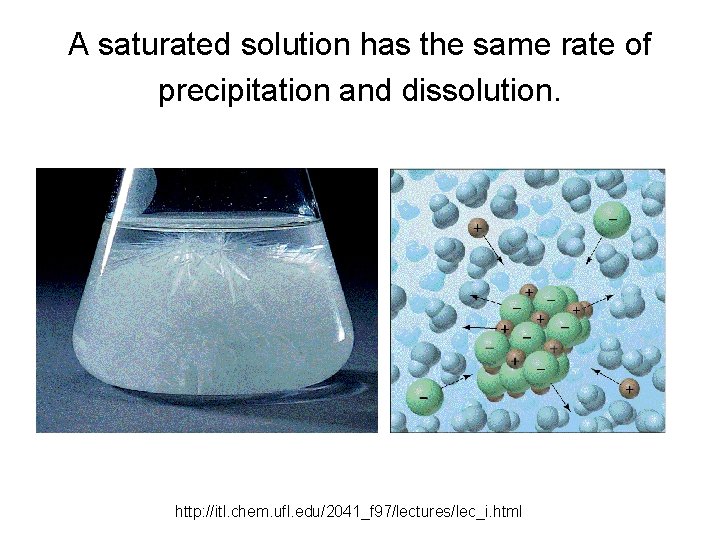
A saturated solution has the same rate of precipitation and dissolution. http: //itl. chem. ufl. edu/2041_f 97/lectures/lec_i. html
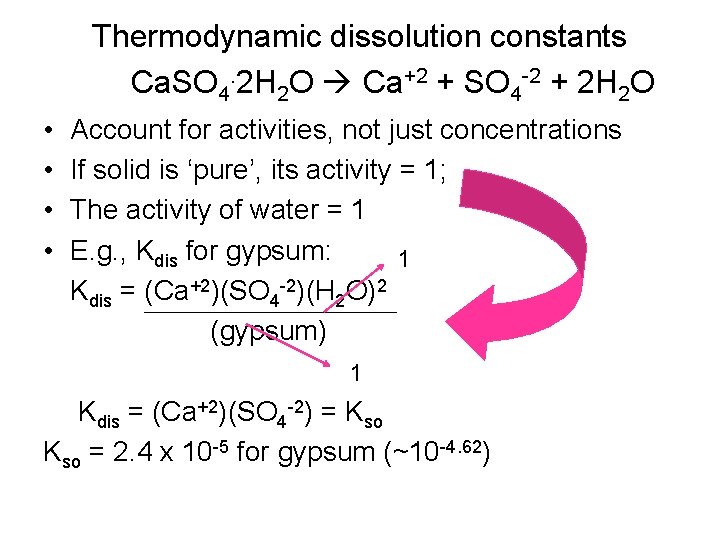
Thermodynamic dissolution constants Ca. SO 4. 2 H 2 O Ca+2 + SO 4 -2 + 2 H 2 O • • Account for activities, not just concentrations If solid is ‘pure’, its activity = 1; The activity of water = 1 E. g. , Kdis for gypsum: 1 Kdis = (Ca+2)(SO 4 -2)(H 2 O)2 (gypsum) 1 Kdis = (Ca+2)(SO 4 -2) = Kso = 2. 4 x 10 -5 for gypsum (~10 -4. 62)
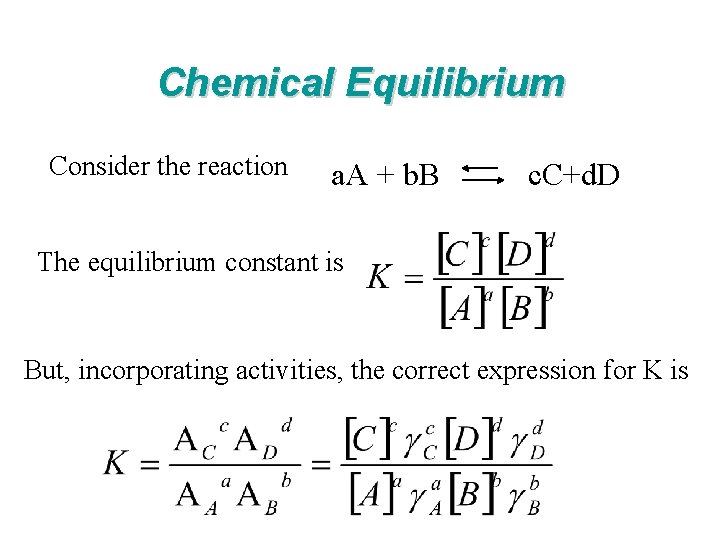
Chemical Equilibrium Consider the reaction a. A + b. B c. C+d. D The equilibrium constant is But, incorporating activities, the correct expression for K is
- Slides: 10