Dissolution 1 Objectives l l Differentiate between different
Dissolution 1

Objectives l l Differentiate between different dissolution apparatus. dissolution test design. Identify different sources of errors in dissolution. Differentiate between media used in different dosage form dissolution tests. 2

Dissolution Definition l l Generally: The rate by which a solid substance enters the solvent phase to yield a solution. Pharmaceutically: The release rate of drug from the dosage form. 3
Importance l Plays an important role in screening formulations, testing oral bioavailability and proving oral bioequivalence. 4

Why dissolution? l Dissolution tests are done at different points during the product development: l Dissolution studies to compare different formulations (preclinical). l Optimize the final formula during phase I and II. l Continues to be a QC test. l If a modified release formula is to be developed, dissolution test should be performed. l If a generic drug is to be approved after the patent expiration, dissolution test should be performed. 5

Dissolution l l Dissolution is related to disintegration, active ingredient solubility, and particle size… ext Disintegration generally reflects the effects of excipients, formulation and the manufacturing processes variables. 6

Dissolution Apparatus l USP recognizes 7 dissolution apparatus l l l Apparatus I (basket) Apparatus II (paddle) Apparatus III (reciprocating cylinder) Apparatus IV (flow-through cell) Apparatus V (paddle over disc) Apparatus VI (cylinder) 7

Apparatus I and II l Apparatus I and II are the most widely used ones. l l l Simple Adequately standardized designs are available Supported by a wider experience of experimental use than the other types. Apparatus I and II are described in USP. Deviation form USP guidelines is accepted but need to be R………. . and V……. . . 8
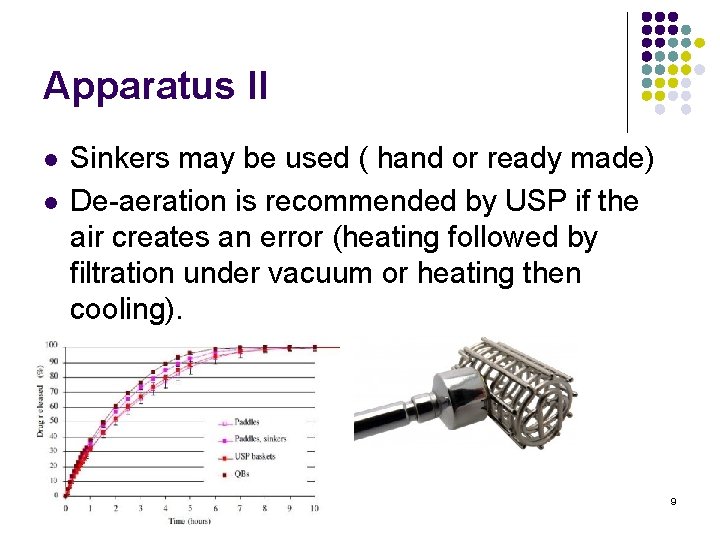
Apparatus II l l Sinkers may be used ( hand or ready made) De-aeration is recommended by USP if the air creates an error (heating followed by filtration under vacuum or heating then cooling). 9
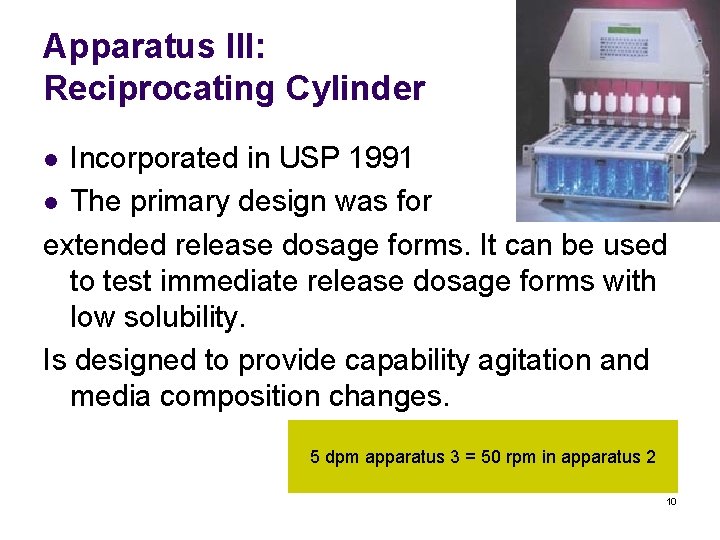
Apparatus III: Reciprocating Cylinder Incorporated in USP 1991 l The primary design was for extended release dosage forms. It can be used to test immediate release dosage forms with low solubility. Is designed to provide capability agitation and media composition changes. l 5 dpm apparatus 3 = 50 rpm in apparatus 2 10

11
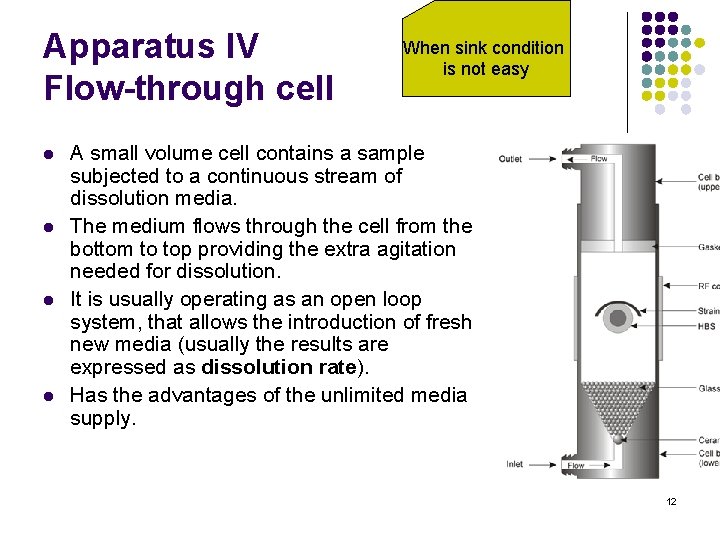
Apparatus IV Flow-through cell l l When sink condition is not easy A small volume cell contains a sample subjected to a continuous stream of dissolution media. The medium flows through the cell from the bottom to top providing the extra agitation needed for dissolution. It is usually operating as an open loop system, that allows the introduction of fresh new media (usually the results are expressed as dissolution rate). Has the advantages of the unlimited media supply. 12

Apparatus IV l l l l Suitable for drugs with limited solubility. Used for tablets, sugar coated tablets, suppositories, soft gel capsules, powders, granules, semisolids and implants. The data is cumulative. The typical volume is 500 -4000 m. L Advantage: providing sink conditions Generating rapid p. H change during the test. Continuous sampling Unlimited solvent volume 13

Flow-through cell 14
15
What are the important control parameters ? 16
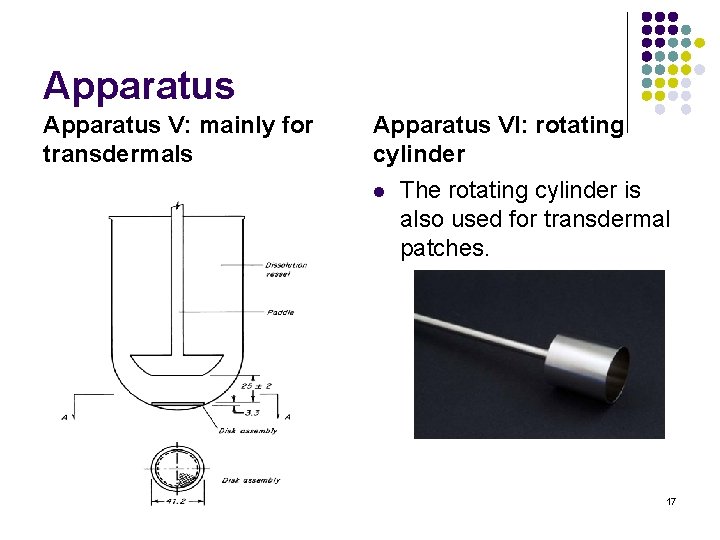
Apparatus V: mainly for transdermals Apparatus VI: rotating cylinder l The rotating cylinder is also used for transdermal patches. 17

Non Compendial Equipment l l Although compendial method should be the first choice, non-compendial methods can be used with enough justification. This may include rotating bottle, mini paddle, mega paddle, unique cell design for apparatus 4 or………. 18

Qualification l l Design Instillation Operation Performance To ensure that the equipment is fit for its intended purpose 19

Qualification Design l The dosage form and the delivery method will dictate the apparatus to be used at least initially. e. g. the beaded product, suppositories, chewable tablets. 20
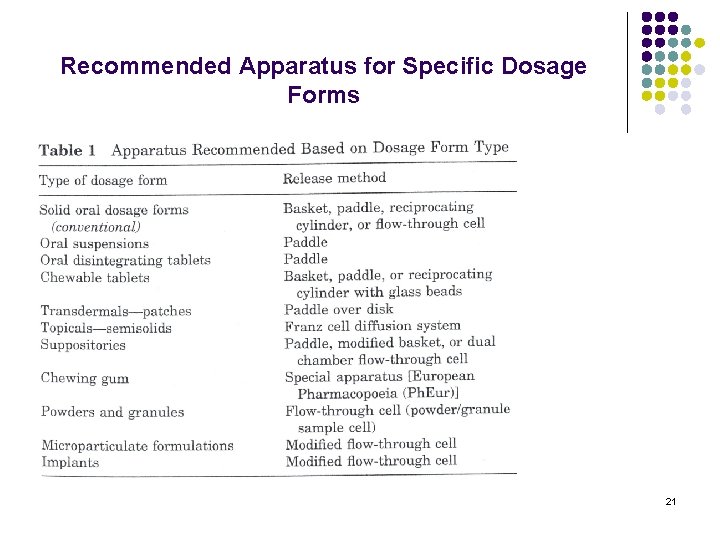
Recommended Apparatus for Specific Dosage Forms 21

Qualification Instillation l The equipment should be set appropriately, with all safety issues addressed. The analyst should verify that the instrument works within the specified range of tolerance. (electric power, viscosity. . 22

Qualification Operational qualification: l During operation the analyst should assess if the equipment works as specified: the water bath, the shaft speed… 23

Qualification Performance qualifications l To ensure that the system is in a normal operation environment reproducing designed set of tasks (reproducibility of the data), the performance qualifications should be tested frequently by the standard tablets. 24

Sources of errors l l There are many sources of errors and a formulator should be able to identify them. High variability proves that the method is not robust and this can cause difficulty in discriminating between formulations. High variability can be caused by equipment effect or formulation effect. 25
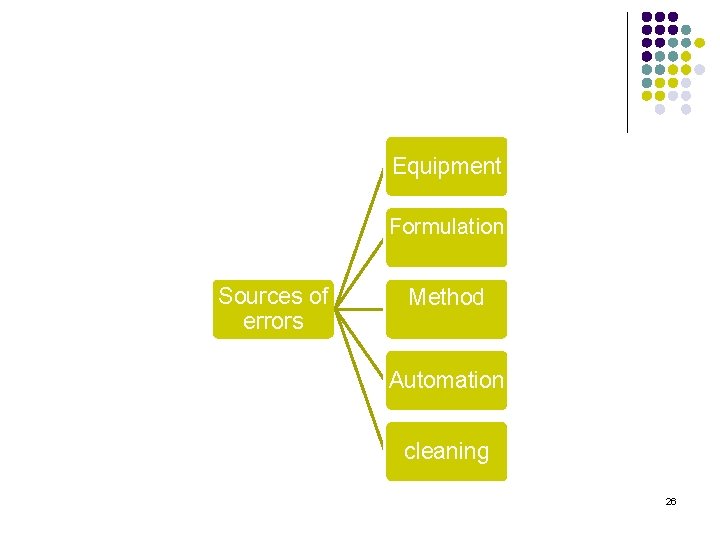
Equipment Formulation Sources of errors Method Automation cleaning 26

Sources of errors l Equipment: 1. 2. 3. l Formulation: l Different formulation requires different apparatus and setting, e. g. coated tablets may stick to the vessels. 27

Sources of errors l Method: l l The introduction of the formulation: suspensions, position of the tablet… The media: sampling volume, sink conditions, media with surfactant> 0. 5% has potential of creating errors, bubbles, increase the media viscosity. 28

Sources of errors l Automation: l l Sample lines: Disconnected, not equal length. Air bubbles in the flow cell. Cells need to be cleaned frequently to avoid the build up of drugs, excipients, surfactant, or buffer salts. Cleaning: l Cleaning the instrument and the sample lines appropriately in between runs specially if the instrument is used in testing different formulas. 29

Hydrodynamic Considerations l l Release-related bioavailability correlations problems have been encountered in pharmaceutical development of formulations. Occasionally the dissolution test expected results are not met due to variations involving the hydrodynamics of the test as the dissolution volume, the stirring device and speed. 30
Hydrodynamic Considerations l Further more there are some factors that may affect the dissolution rate as small fluctuation of temperature, by affecting the drug saturation solubility and viscosity as well as the diffusion coefficient (Noyes Whitney equation). 31

How to start l l Sink conditions? The volume of the media is at least 3 times greater than the saturation solubility Which media to run which test? ? A media that does not provide sink conditions can be used with justification, to discriminate between the dosage forms. 32
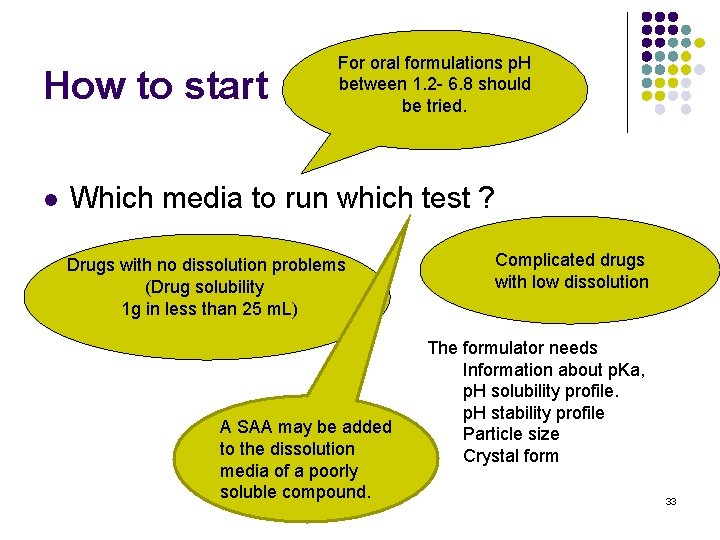
How to start l For oral formulations p. H between 1. 2 - 6. 8 should be tried. Which media to run which test ? Drugs with no dissolution problems (Drug solubility 1 g in less than 25 m. L) A SAA may be added to the dissolution media of a poorly soluble compound. Complicated drugs with low dissolution The formulator needs Information about p. Ka, p. H solubility profile. p. H stability profile Particle size Crystal form 33

Key Operating Parameters l l Media: volume, temperature & deaeration(it is important to deaerate the media, except. . ? (SAA). Sampling time points, depends on l l Dosage form. The purpose of the test. 34

35

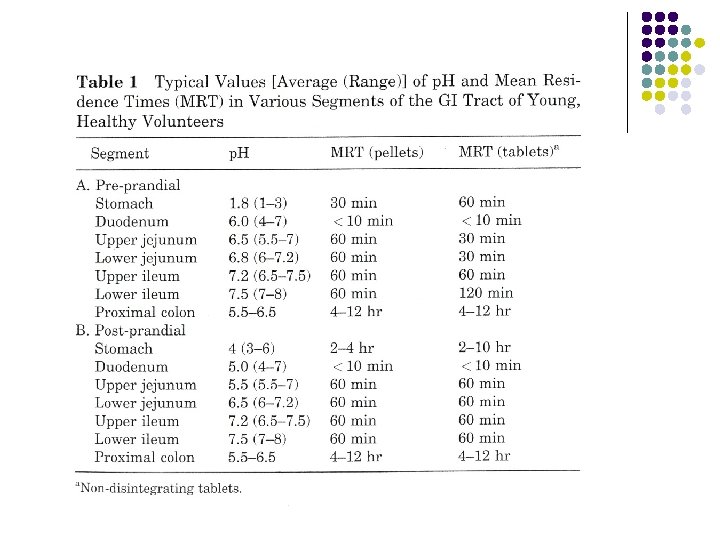
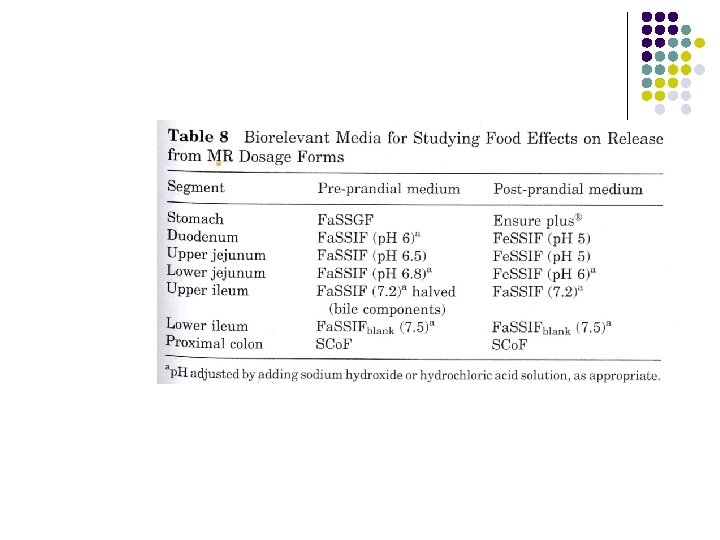
Dissolution media l l Biorelevant Media with some relevance to the in vivo dissolutions conditions for the compound. In vitro- in vivo correlation. p. H , bile salt, osmolarity, food 36

Dissolution media Temp. Standard: 37 0. 5 OC oral 38 0. 5 OC suppositories 32 0. 5 OC topical 37

Misleading single media test Release from 4 commercially available mesalazine (used for chronic inflammatory conditions like ulcerative colitis) product in single media top p. H 6. 8 (jejunum) and bottom p. H 7. 5 (ileum), all 4 brands are coated.

Few discussion points homework l l l Can organic solvents be used as a media? What about water? Franz diffusion cells, use, challenges, modifications. (5 references). 41

references l Pharmaceutical Dissolution testing by Jennifer Dressman, Tayalor & Francis 42
- Slides: 42