DISSOCIATION OF IONS Chemistry 11 Solution Chemistry 3




- Slides: 11

DISSOCIATION OF IONS Chemistry 11 – Solution Chemistry 3

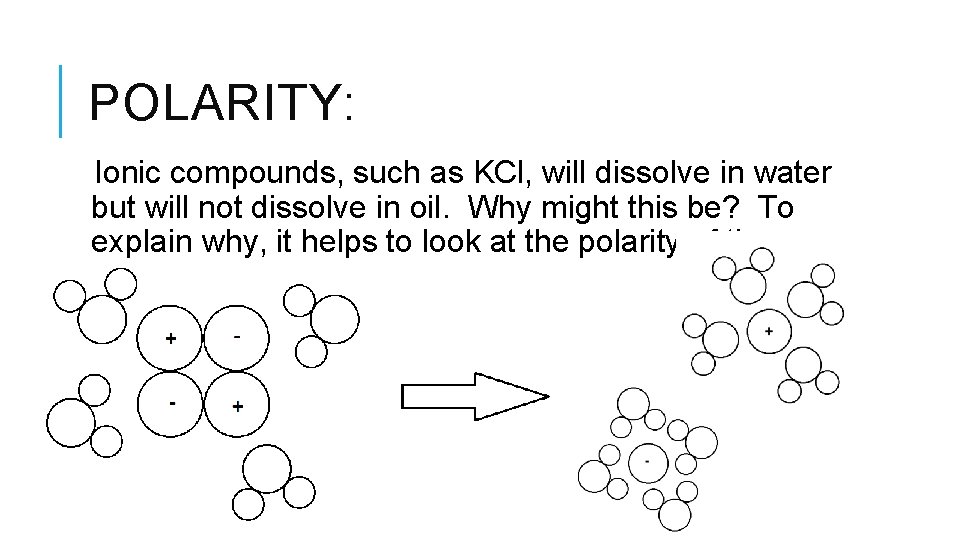
POLARITY: Ionic compounds, such as KCl, will dissolve in water but will not dissolve in oil. Why might this be? To explain why, it helps to look at the polarity of the molecules:

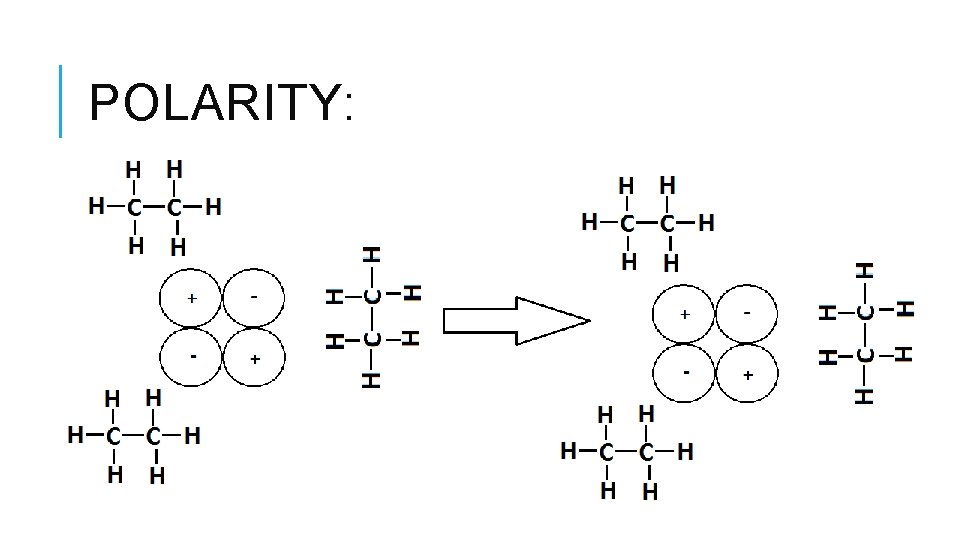
POLARITY:

IN SUMMARY: Polar or ionic solutes tend to dissolve in polar solvents. Nonpolar solutes tend to dissolve in nonpolar solvents. Like Dissolves Like

DISSOCIATION OF IONS: When ionic solids are put in to polar liquids, they dissolve, or dissociate into their ions: KI(s) K+(aq) + I-(aq) Dissociation is not a chemical reaction. Because not all ionic solids have a 1: 1 ratio of cations and anions, their concentrations will not be the same.

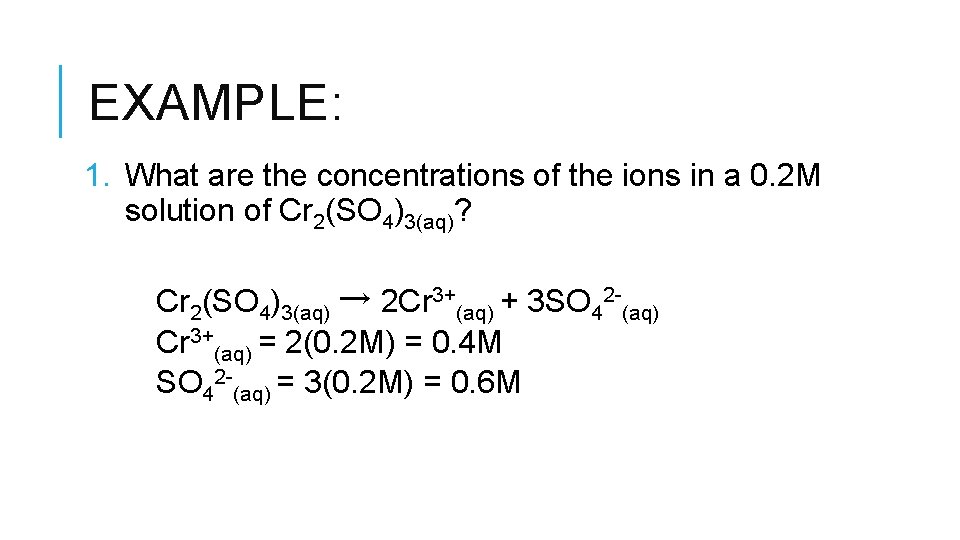
EXAMPLE: 1. What are the concentrations of the ions in a 0. 2 M solution of Cr 2(SO 4)3(aq)? Cr 2(SO 4)3(aq) → 2 Cr 3+(aq) + 3 SO 42 -(aq) Cr 3+(aq) = 2(0. 2 M) = 0. 4 M SO 42 -(aq) = 3(0. 2 M) = 0. 6 M

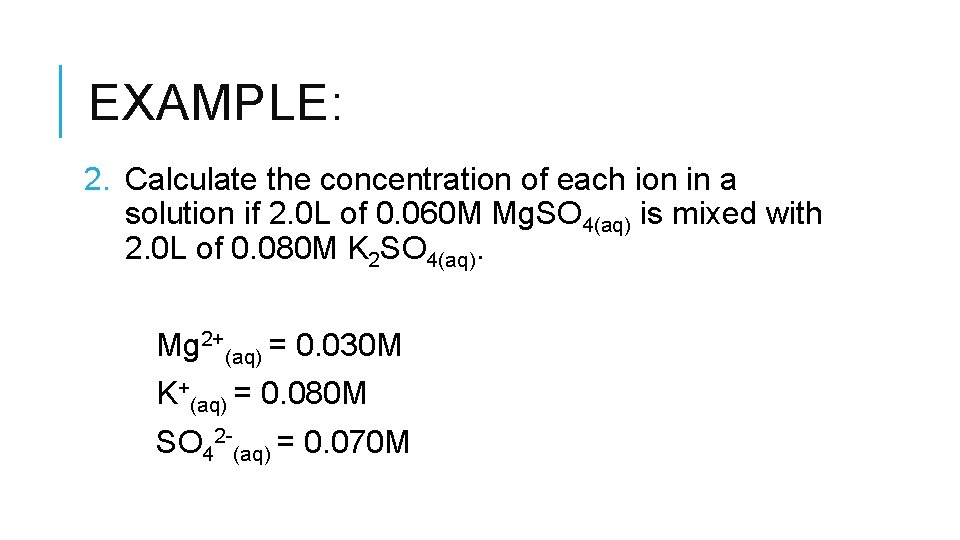
EXAMPLE: 2. Calculate the concentration of each ion in a solution if 2. 0 L of 0. 060 M Mg. SO 4(aq) is mixed with 2. 0 L of 0. 080 M K 2 SO 4(aq). Mg 2+(aq) = 0. 030 M K+(aq) = 0. 080 M SO 42 -(aq) = 0. 070 M

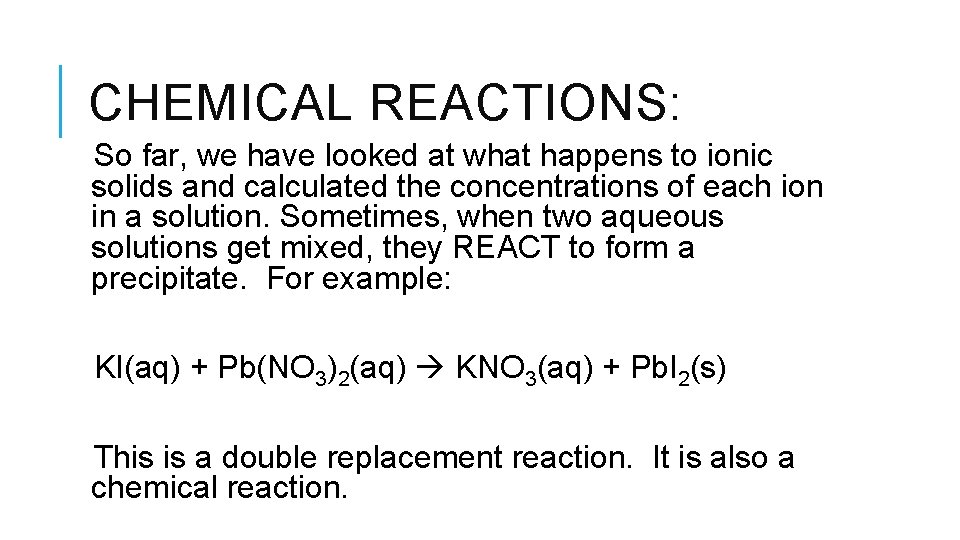
CHEMICAL REACTIONS: So far, we have looked at what happens to ionic solids and calculated the concentrations of each ion in a solution. Sometimes, when two aqueous solutions get mixed, they REACT to form a precipitate. For example: KI(aq) + Pb(NO 3)2(aq) KNO 3(aq) + Pb. I 2(s) This is a double replacement reaction. It is also a chemical reaction.

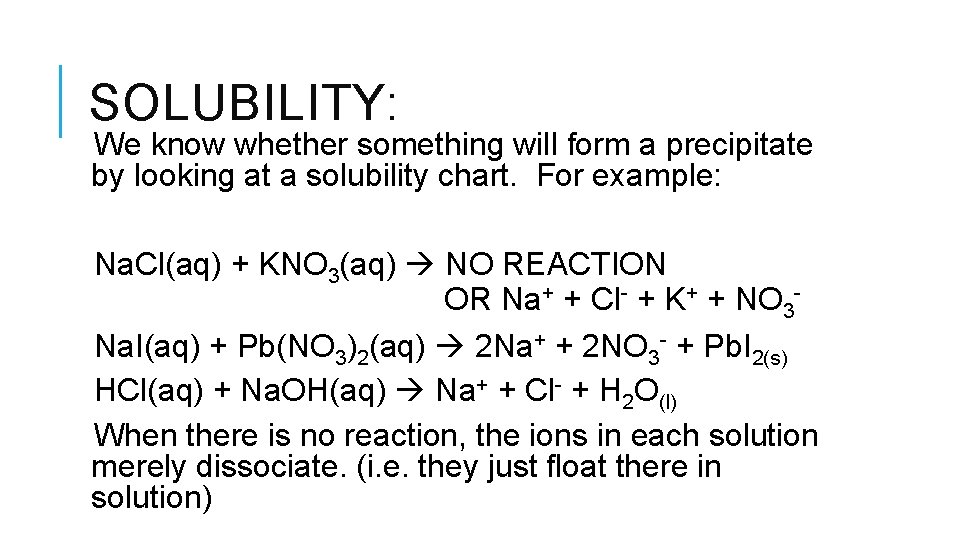
SOLUBILITY: We know whether something will form a precipitate by looking at a solubility chart. For example: Na. Cl(aq) + KNO 3(aq) NO REACTION OR Na+ + Cl- + K+ + NO 3 Na. I(aq) + Pb(NO 3)2(aq) 2 Na+ + 2 NO 3 - + Pb. I 2(s) HCl(aq) + Na. OH(aq) Na+ + Cl- + H 2 O(l) When there is no reaction, the ions in each solution merely dissociate. (i. e. they just float there in solution)

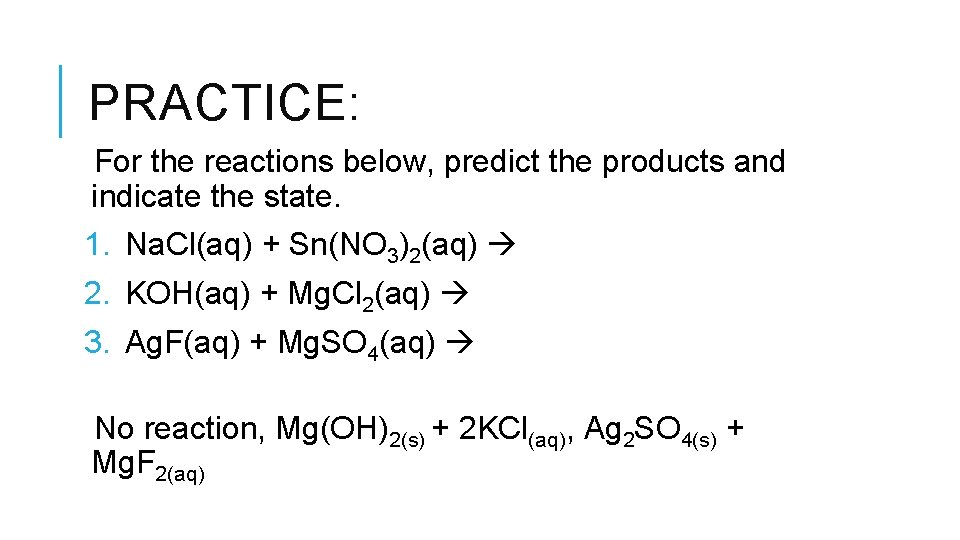
PRACTICE: For the reactions below, predict the products and indicate the state. 1. Na. Cl(aq) + Sn(NO 3)2(aq) 2. KOH(aq) + Mg. Cl 2(aq) 3. Ag. F(aq) + Mg. SO 4(aq) No reaction, Mg(OH)2(s) + 2 KCl(aq), Ag 2 SO 4(s) + Mg. F 2(aq)

ASSIGNMENT Worksheet