Dissecting the Atom A Look at Chemical Symbols

Dissecting the Atom: A Look at Chemical Symbols

First, a review… • An atom is the smallest part of an element that still behaves like the element. • A molecule is a particle that is composed of two or more atoms held together by a chemical bond.
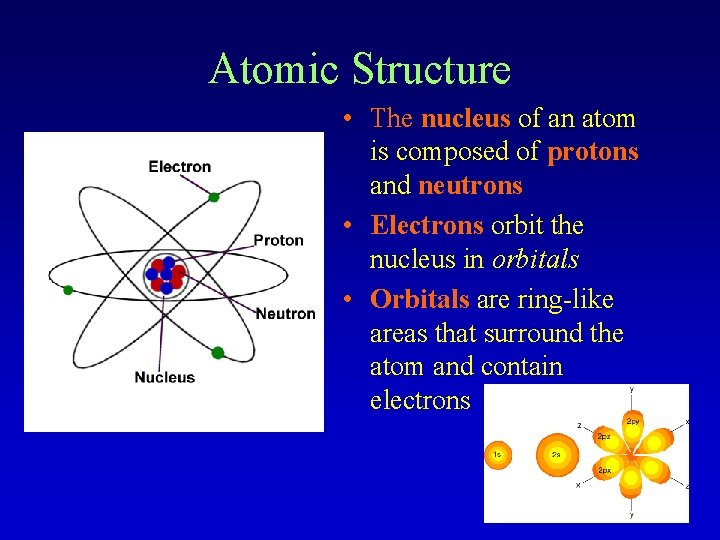
Atomic Structure • The nucleus of an atom is composed of protons and neutrons • Electrons orbit the nucleus in orbitals • Orbitals are ring-like areas that surround the atom and contain electrons

Charges The subatomic particles in an atom have different charges: • Protons have a POSITIVE charge (p+) • Neutrons have a NEUTRAL charge (n 0) • Electrons have a NEGATIVE charge (e-)

Looking at the Periodic Table Chemical Symbols – There about a dozen common elements that have a single capitalized letter for their symbol – The rest, that have permanent names have two letters. • the first is capitalized and the second is lower case. – Some elements have symbols from their Latin names. – Ten of the elements have symbols from their Latin or German names.
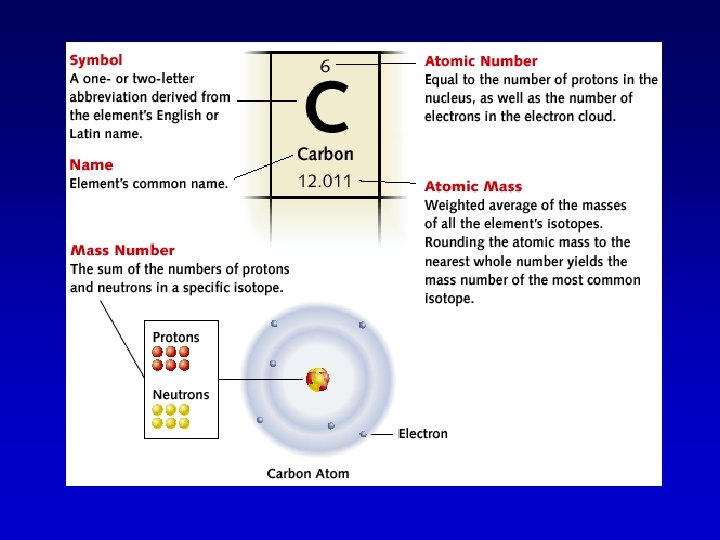
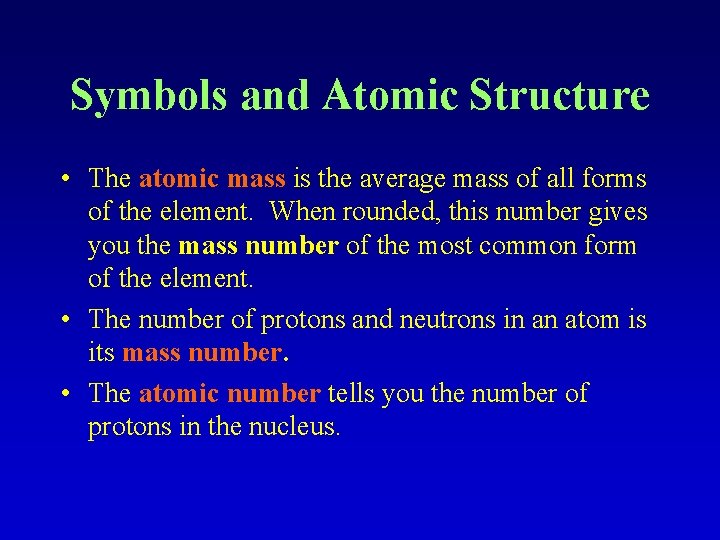
Symbols and Atomic Structure • The atomic mass is the average mass of all forms of the element. When rounded, this number gives you the mass number of the most common form of the element. • The number of protons and neutrons in an atom is its mass number. • The atomic number tells you the number of protons in the nucleus.

Key Points to remember: • Atomic numbers are whole numbers • Mass numbers are whole numbers • The atomic mass is not a whole number (because it is an average mass of all forms of the element)
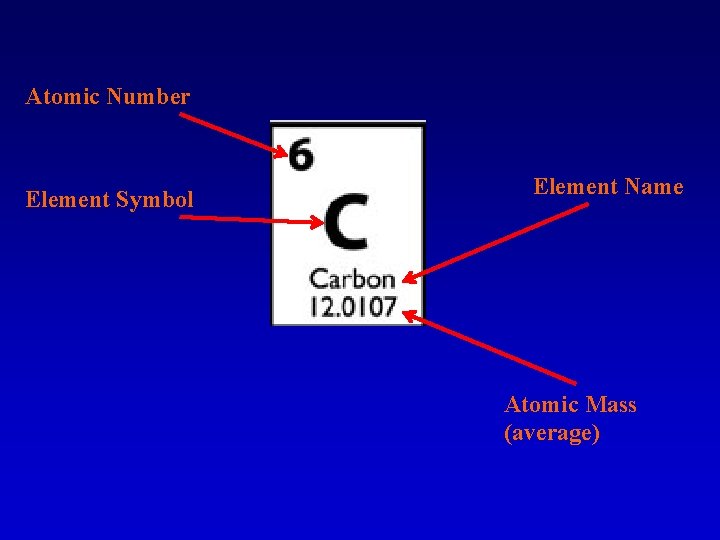
Atomic Number Element Symbol Element Name Atomic Mass (average)

How do you find…? 1. The number of protons in an atom? 2. The number of neutrons in an atom? 3. The number of electrons in an atom? 4. The mass number? 1. The atomic number 2. Mass number – atomic number 3. The atomic number 4. Round the atomic mass to the nearest whole number OR add up the number of protons and neutrons
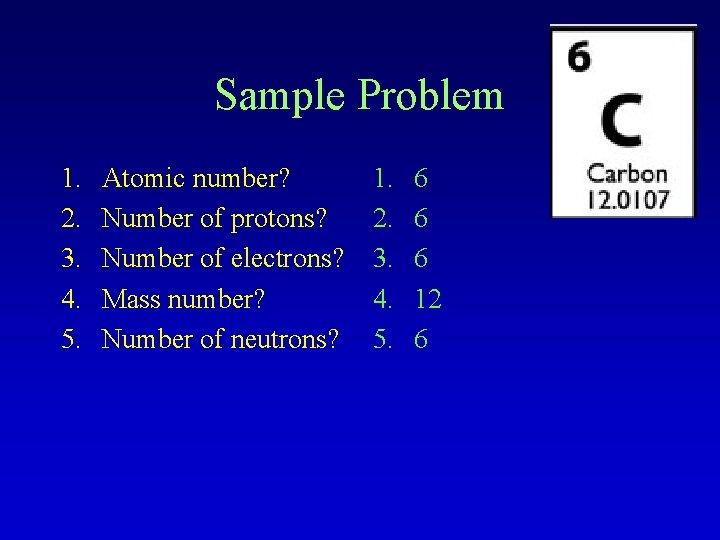
Sample Problem 1. 2. 3. 4. 5. Atomic number? Number of protons? Number of electrons? Mass number? Number of neutrons? 1. 2. 3. 4. 5. 6 6 6 12 6
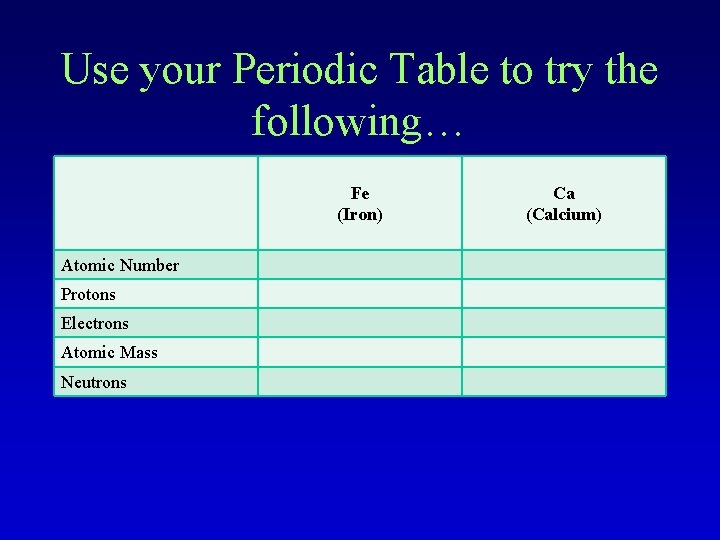
Use your Periodic Table to try the following… Fe (Iron) Atomic Number Protons Electrons Atomic Mass Neutrons Ca (Calcium)
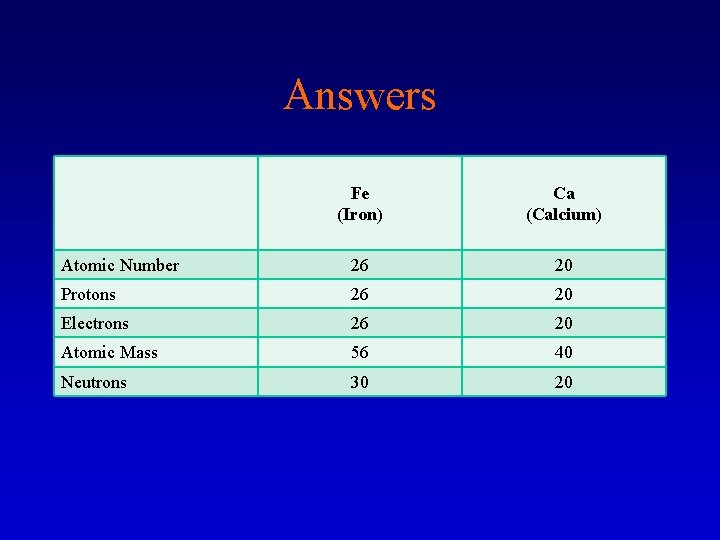
Answers Fe (Iron) Ca (Calcium) Atomic Number 26 20 Protons 26 20 Electrons 26 20 Atomic Mass 56 40 Neutrons 30 20
- Slides: 13