Displacement Reactions Displacement Reactions Lets look at the






- Slides: 11

Displacement Reactions

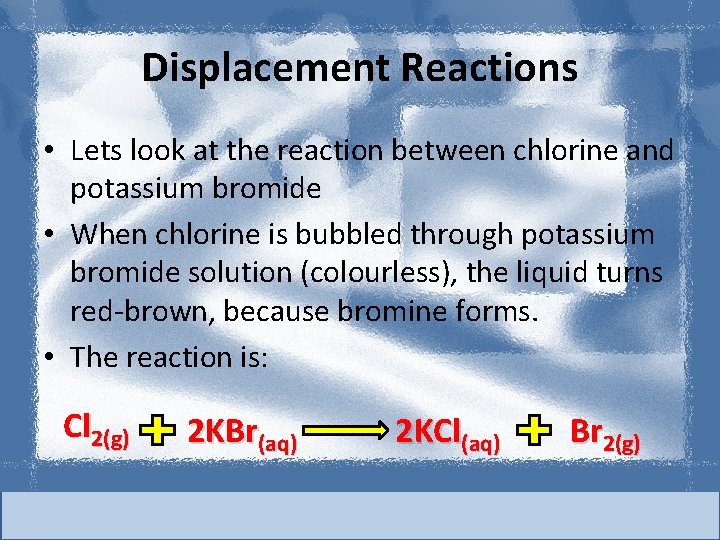
Displacement Reactions • Lets look at the reaction between chlorine and potassium bromide • When chlorine is bubbled through potassium bromide solution (colourless), the liquid turns red-brown, because bromine forms. • The reaction is: Cl 2(g) 2 KBr(aq) 2 KCl(aq) Br 2(g)

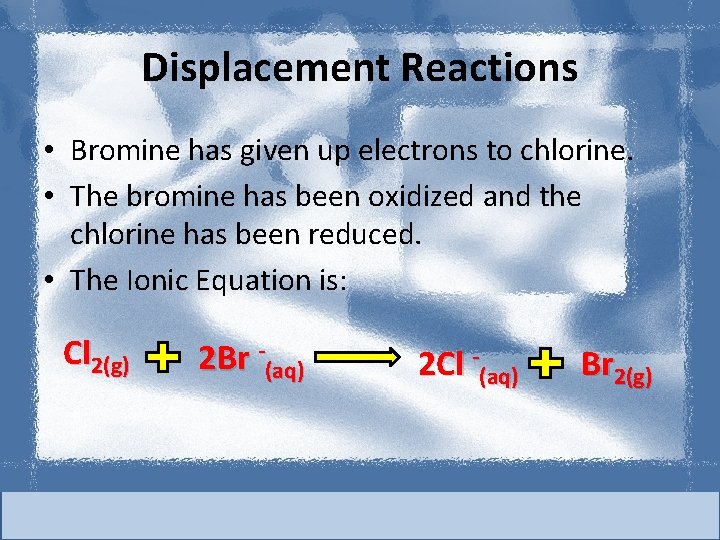
Displacement Reactions • Bromine has given up electrons to chlorine. • The bromine has been oxidized and the chlorine has been reduced. • The Ionic Equation is: Cl 2(g) 2 Br -(aq) 2 Cl -(aq) Br 2(g)

Displacement Reactions • In effect chlorine has pushed bromine out of the potassium compound and taken its place. • It has displaced bromine. • Redox reactions like these, where one element displaces another from a dissolved compound (solution), are called displacement reactions.

Displacement of One Metal by Another. • An iron nail is placed in copper II sulfate solution. • Soon copper appears on the nail and the solution turns green. • Here iron and copper are competing to be the compound in solution. Iron wins. • It displaces copper from copper II sulfate solution. A green iron II sulfate solution is formed.

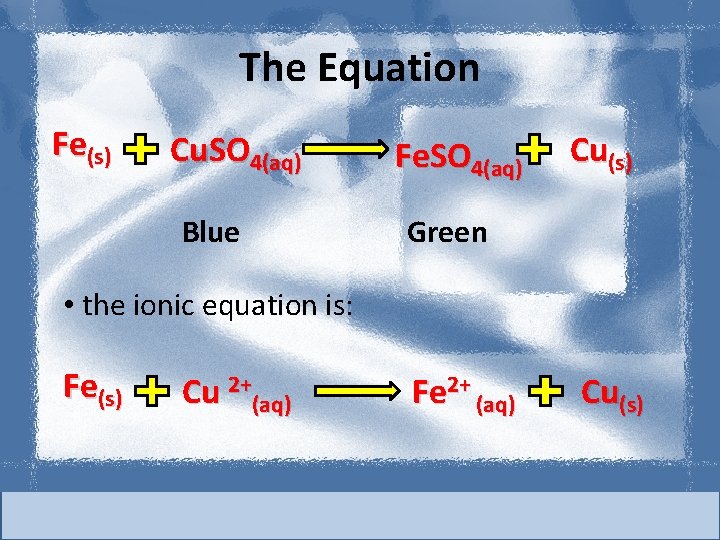
The Equation Fe(s) Cu. SO 4(aq) Blue Fe. SO 4(aq) Cu(s) Green • the ionic equation is: Fe(s) Cu 2+(aq) Fe 2+ (aq) Cu(s)

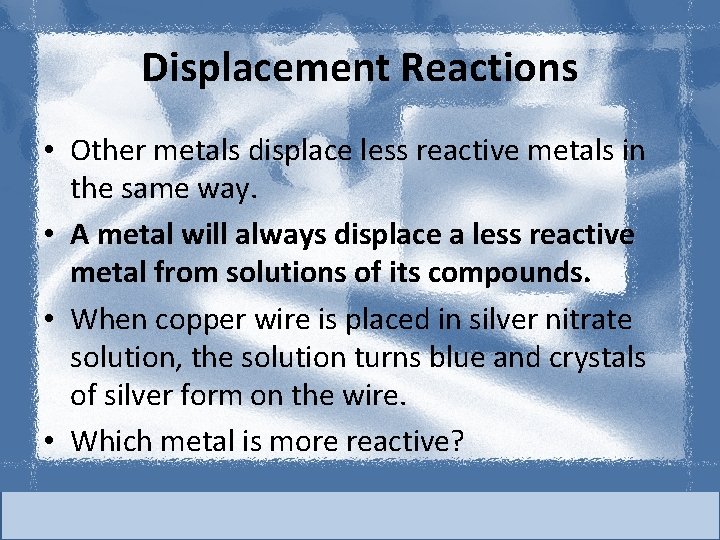
Displacement Reactions • Other metals displace less reactive metals in the same way. • A metal will always displace a less reactive metal from solutions of its compounds. • When copper wire is placed in silver nitrate solution, the solution turns blue and crystals of silver form on the wire. • Which metal is more reactive?

Remember the Activity Series • Potassium > Sodium > Lithium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > (hydrogen) > Copper > Silver > Gold • Copper is more reactive. Cu(s) Ag. NO 3(aq) Cu(NO 3)2(aq) Ag(s)

Try This…. • What would you see if you added zinc to copper sulfate solution? • Hint zinc sulfate is colourless.

Now Try This…… • Tin does not react with iron II oxide. But it reduces lead II oxide to lead. • Arrange tin, iron and lead in order of decreasing reactivity. • Iron > tin > lead

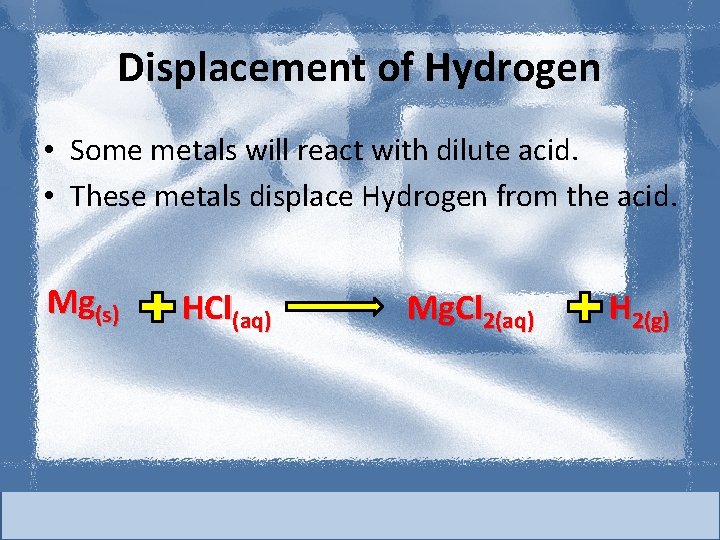
Displacement of Hydrogen • Some metals will react with dilute acid. • These metals displace Hydrogen from the acid. Mg(s) HCl(aq) Mg. Cl 2(aq) H 2(g)
 Look up look down look around
Look up look down look around Single displacement vs double displacement
Single displacement vs double displacement Reactivity series and displacement reactions
Reactivity series and displacement reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Reduction half reaction
Reduction half reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Activity 1 picture
Activity 1 picture Picture reading activity
Picture reading activity Activity 1 look at the picture
Activity 1 look at the picture Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia