Dispersion Terminology How do we quantify the spatial






- Slides: 42

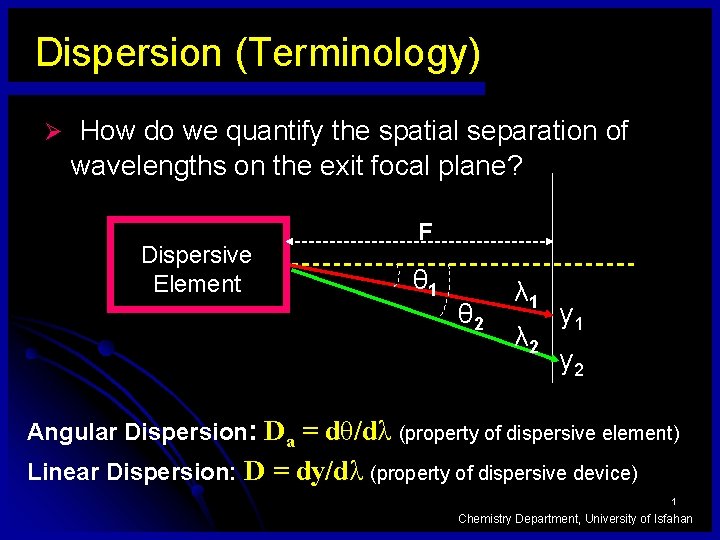
Dispersion (Terminology) Ø How do we quantify the spatial separation of wavelengths on the exit focal plane? Dispersive Element F θ 1 θ 2 λ 1 λ 2 y 1 y 2 Angular Dispersion: Da = dθ/dλ (property of dispersive element) Linear Dispersion: D = dy/dλ (property of dispersive device) 1 Chemistry Department, University of Isfahan

More Dispersive Terminology § If dθ is small, it can be shown that: D = F × Da • • • Sin d = dy/F More commonly, we will use: Reciprocal Linear Dispersion (D-1) = 1/D • -typically around 0. 1 - 20 Å/mm in UV/Vis Effective Bandwidth: Δλeff = D-1 × w Slit-width D-1 = 16 Å/mm w = 100 μm Δλeff = 1. 6 Å 2 Chemistry Department, University of Isfahan

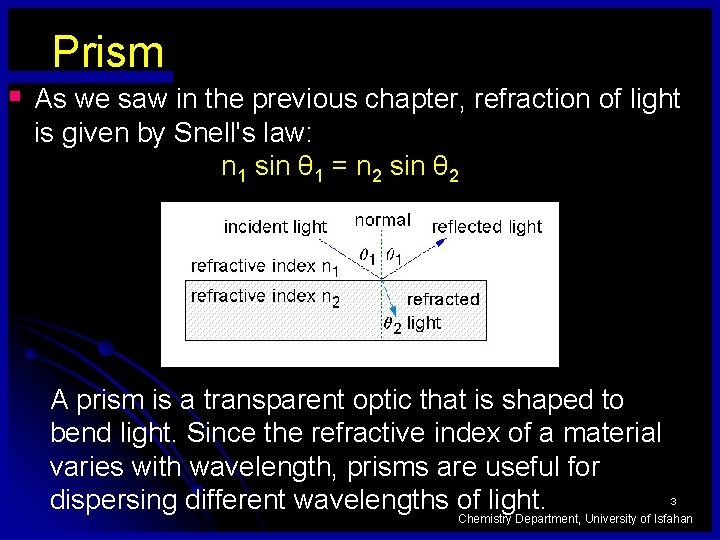
Prism § As we saw in the previous chapter, refraction of light is given by Snell's law: n 1 sin θ 1 = n 2 sin θ 2 A prism is a transparent optic that is shaped to bend light. Since the refractive index of a material varies with wavelength, prisms are useful for dispersing different wavelengths of light. 3 Chemistry Department, University of Isfahan

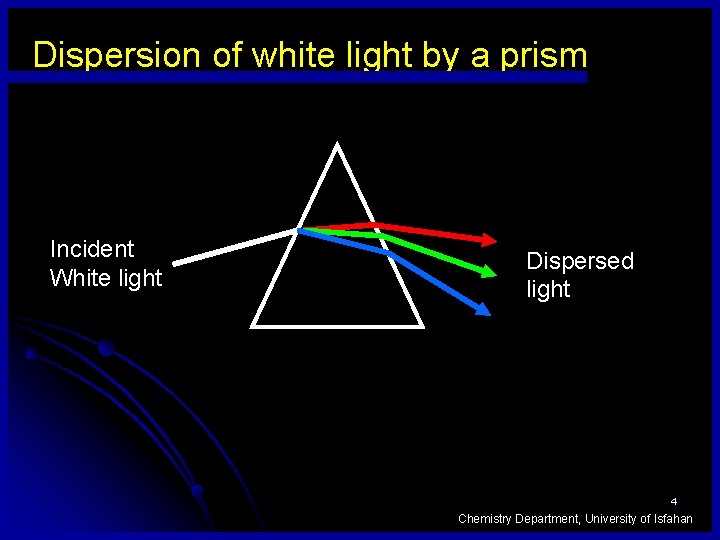
Dispersion of white light by a prism Incident White light Dispersed light 4 Chemistry Department, University of Isfahan

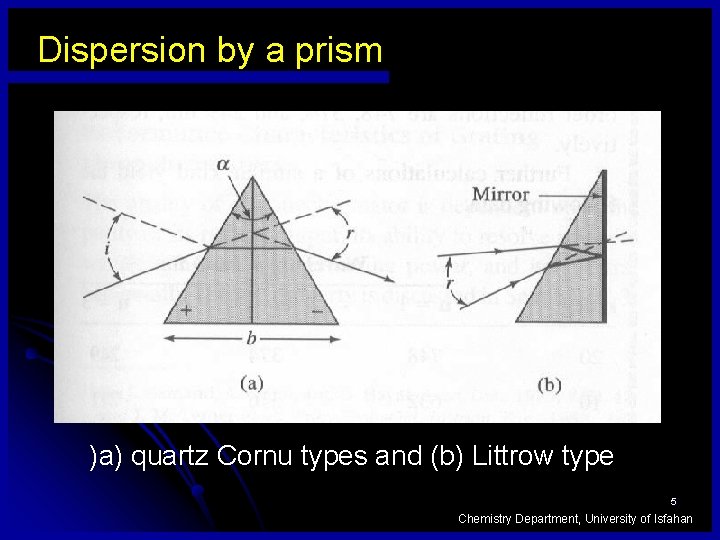
Dispersion by a prism )a) quartz Cornu types and (b) Littrow type 5 Chemistry Department, University of Isfahan

6

Rainbow 7 Chemistry Department, University of Isfahan

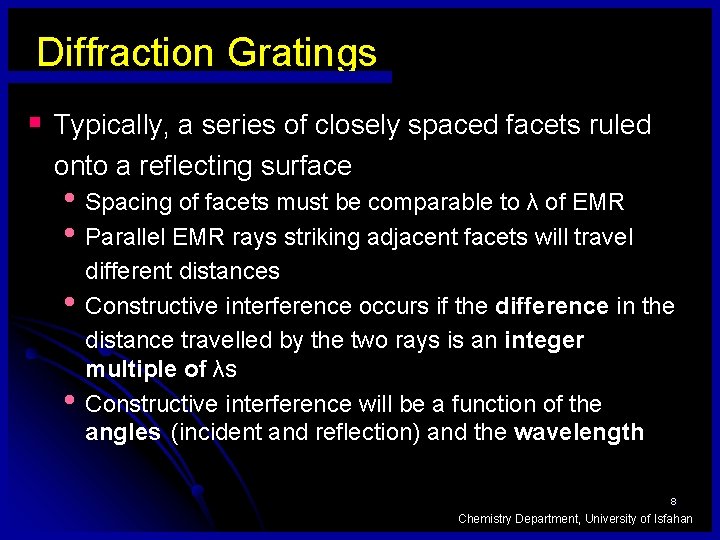
Diffraction Gratings § Typically, a series of closely spaced facets ruled onto a reflecting surface • Spacing of facets must be comparable to λ of EMR • Parallel EMR rays striking adjacent facets will travel • • different distances Constructive interference occurs if the difference in the distance travelled by the two rays is an integer multiple of λs Constructive interference will be a function of the angles (incident and reflection) and the wavelength 8 Chemistry Department, University of Isfahan

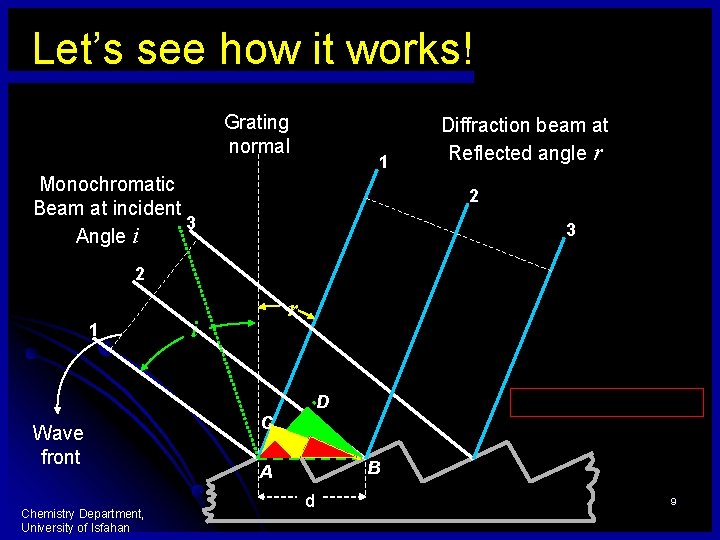
Let’s see how it works! Grating normal 1 Monochromatic Beam at incident 3 Angle i Diffraction beam at Reflected angle r 2 3 2 1 r i D Wave front Chemistry Department, University of Isfahan C B A d 9

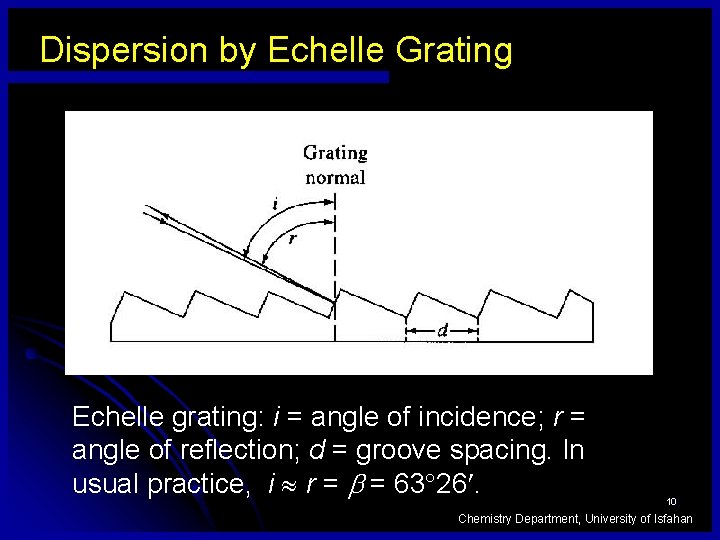
Dispersion by Echelle Grating Echelle grating: i = angle of incidence; r = angle of reflection; d = groove spacing. In usual practice, i r = = 63 26. 10 Chemistry Department, University of Isfahan

Echelle Grating § Provides high dispersion and high resolution § Difference with echellette grating • Its blaze angle is significantly greater • The short side of the blaze is used rather than the long • • side which is used in echellette grating The grating is relatively coarse, having typically 300 or fewer grooves per mm for UV radiation The angle of reflection r is much higher in the echelle grating than the echellette and approaches the angle of incidence i, r i= 11 Chemistry Department, University of Isfahan

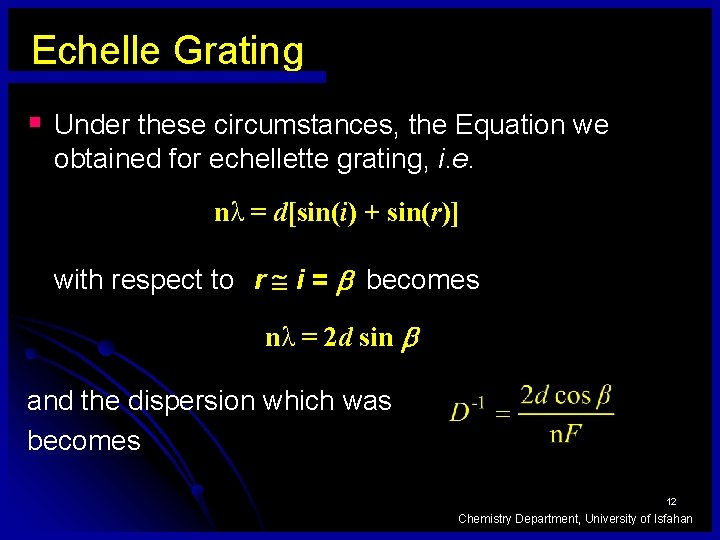
Echelle Grating § Under these circumstances, the Equation we obtained for echellette grating, i. e. nλ = d[sin(i) + sin(r)] with respect to r i = becomes nλ = 2 d sin and the dispersion which was becomes 12 Chemistry Department, University of Isfahan

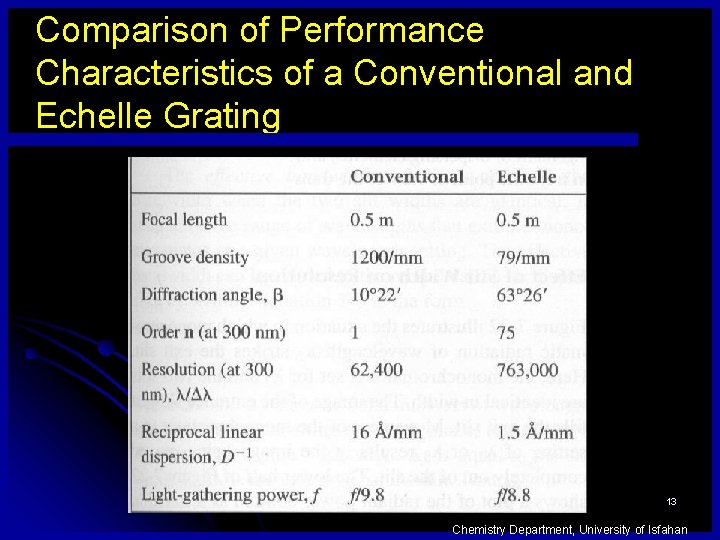
Comparison of Performance Characteristics of a Conventional and Echelle Grating 13 Chemistry Department, University of Isfahan

Performance Specifications The dispersion is usually given in nanometers per millimeter, where the slit width is expressed in mm. stray light: light transmitted by the monochromator at wavelengths outside of the chosen wavelength and bandpass. high efficiency: maximize the ability to detect low light levels. Resolution is usually of secondary importance since emission spectra rarely have peaks with linewidths less than 5 nm. 14 Chemistry Department, University of Isfahan

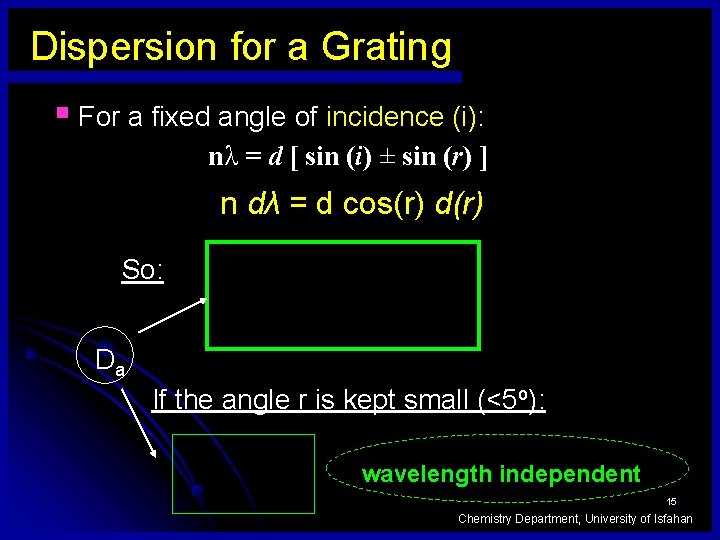
Dispersion for a Grating § For a fixed angle of incidence (i): nλ = d [ sin (i) ± sin (r) ] n dλ = d cos(r) d(r) So: Da If the angle r is kept small (<5 o): wavelength independent 15 Chemistry Department, University of Isfahan

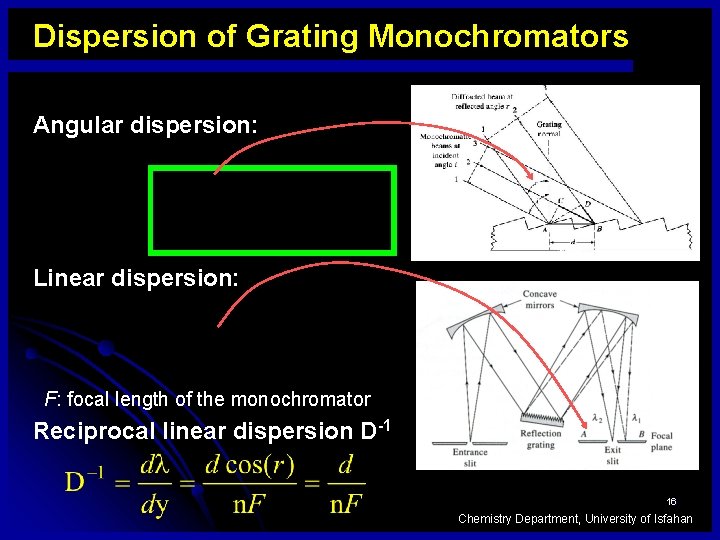
Dispersion of Grating Monochromators Angular dispersion: Linear dispersion: F: focal length of the monochromator Reciprocal linear dispersion D-1 16 Chemistry Department, University of Isfahan

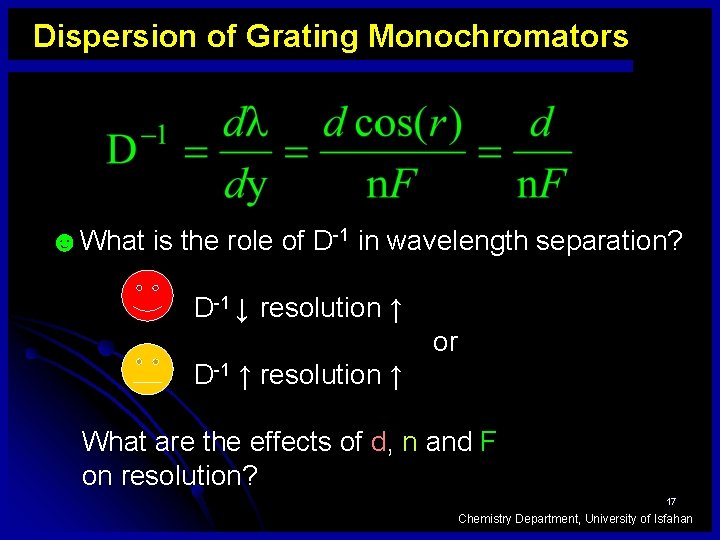
Dispersion of Grating Monochromators ☻What is the role of D-1 in wavelength separation? D-1 ↓ resolution ↑ or D-1 ↑ resolution ↑ What are the effects of d, n and F on resolution? 17 Chemistry Department, University of Isfahan

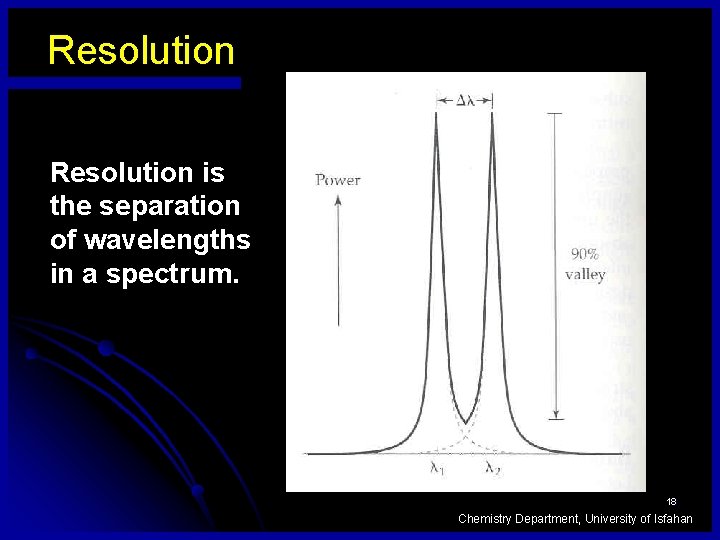
Resolution is the separation of wavelengths in a spectrum. 18 Chemistry Department, University of Isfahan

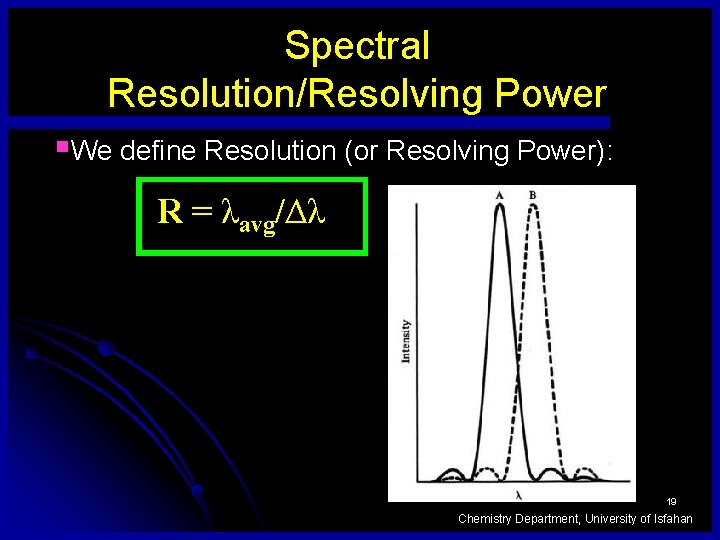
Spectral Resolution/Resolving Power §We define Resolution (or Resolving Power): R = λavg/Δλ 19 Chemistry Department, University of Isfahan

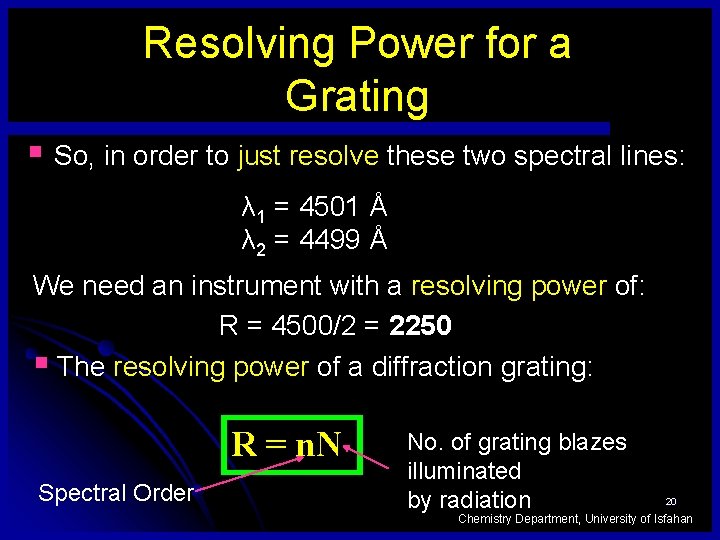
Resolving Power for a Grating § So, in order to just resolve these two spectral lines: λ 1 = 4501 Å λ 2 = 4499 Å We need an instrument with a resolving power of: R = 4500/2 = 2250 § The resolving power of a diffraction grating: R = n. N Spectral Order No. of grating blazes illuminated by radiation 20 Chemistry Department, University of Isfahan

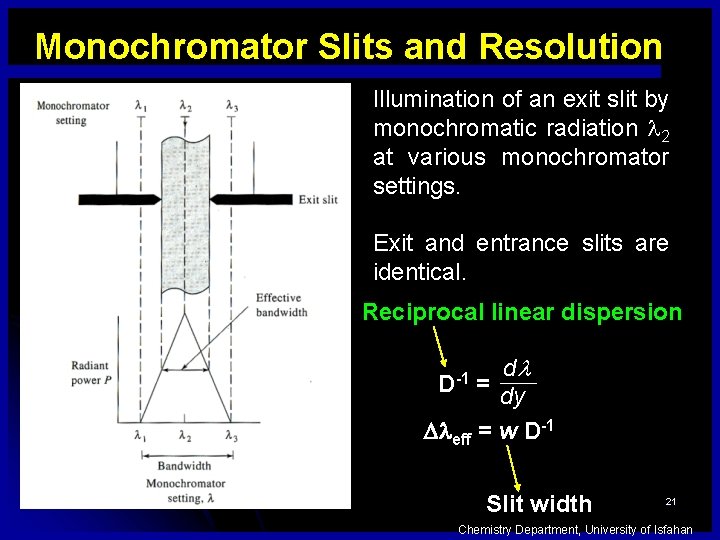
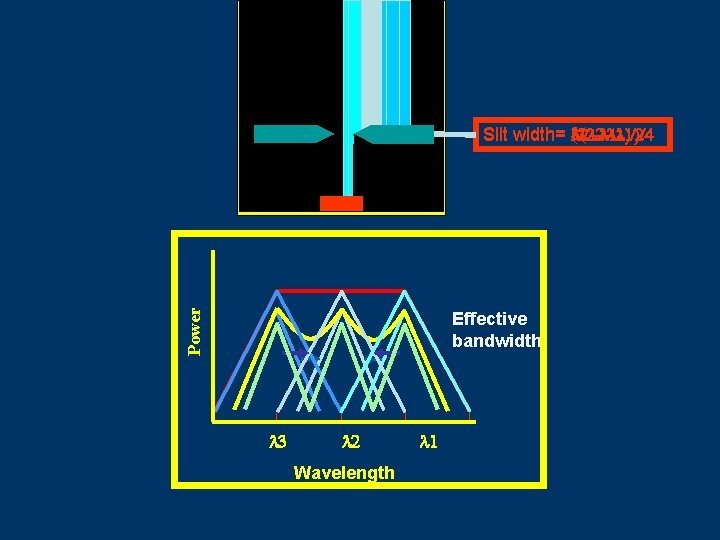
Monochromator Slits and Resolution Illumination of an exit slit by monochromatic radiation 2 at various monochromator settings. Exit and entrance slits are identical. Reciprocal linear dispersion dl dy D eff = w D-1 = Slit width 21 Chemistry Department, University of Isfahan

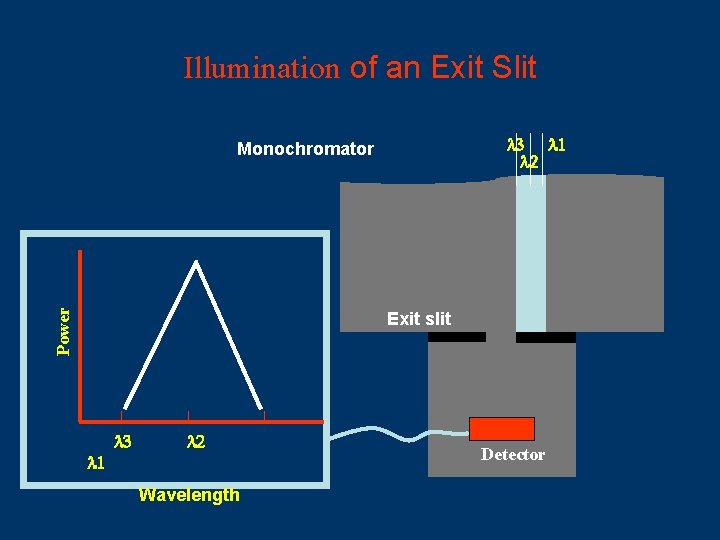
Illumination of an Exit Slit 3 1 2 Power Monochromator Exit slit 1 3 2 Wavelength Detector

Power Slit width= 3( 2 - 1)/4 ( 2 - 1)/2 2 - 1 Effective bandwidth 3 2 Wavelength 1

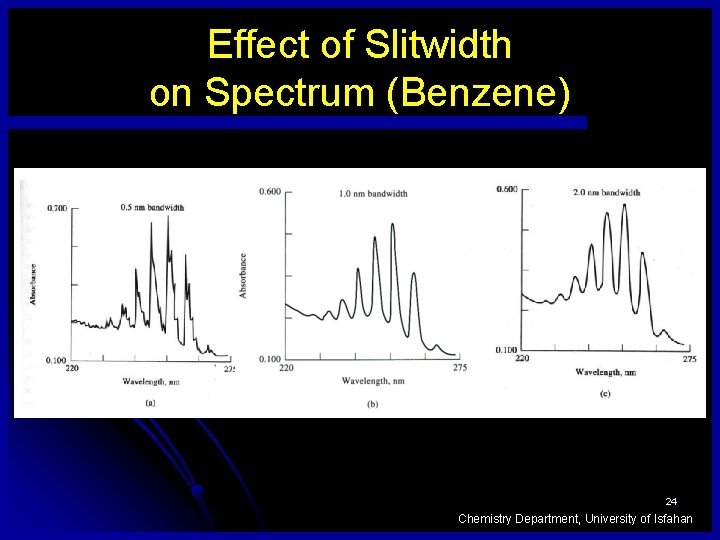
Effect of Slitwidth on Spectrum (Benzene) 24 Chemistry Department, University of Isfahan

Slit width • The slit widths are generally variable, and a typical monochromator will have both an entrance and an exit slit. • The light intensity which passes through a monochromator is approximately proportional to the square of the slit width. • Larger slit widths yield increased signal, what about resolution? 25 Chemistry Department, University of Isfahan

How it all fits together Ø Suppose we want to “just resolve” the following Iron doublet: λ 1 = 3099. 90 Å λ 2 = 3099. 97 Å R= 3099. 935/0. 07 = 44, 000 Suppose that we have a 100 -mm wide grating ruled with 1200 grove/mm ; it has a first-order resolving power of: R = n. N = (1)(100 mm)(1200 gr/mm) R = 120, 000 26 Chemistry Department, University of Isfahan

But, can we really resolve the two lines? Ø Consider, now, the dispersion of the monochromator in which that grating is located: D-1 = 16 Å/mm In order to just resolve the two lines: Δλeff = 0. 07 Å This requires a slitwidth of: Δλeff = D-1 × w w = 0. 07 Å/16 Å/mm = 0. 00438 mm w ≈ 4 μm Chemistry Department, University of Isfahan 27

How Can We Improve Resolution? Ø Decrease Slitwidth (w) -limits light throughput Ø Operate in Higher Spectral Orders -limits light throughput (decreased efficiency) D-1 ∝ 1/n Ø Increase Focal Length -limits light throughput (inverse square law) D-1 ∝ 1/F 28 Chemistry Department, University of Isfahan

Optical instrument Five components 1. a stable source of radiant energy 2. a transparent container for holding the sample 3. a device that isolates a restricted region of the spectrum for measurement 4. a radiation detector 5. a signal processor and Fiber Optics 29 Chemistry Department, University of Isfahan

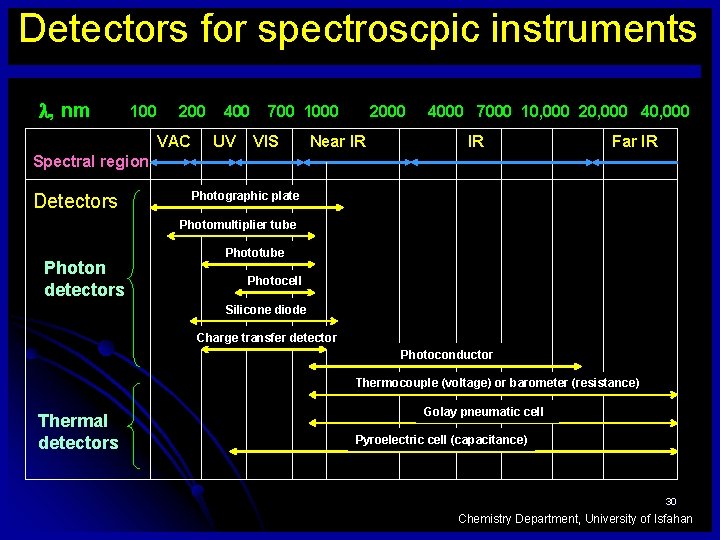
Detectors for spectroscpic instruments , nm 100 200 VAC 400 UV 700 1000 VIS 2000 Near IR 4000 7000 10, 000 20, 000 40, 000 IR Far IR Spectral region Detectors Photographic plate Photomultiplier tube Photon detectors Phototube Photocell Silicone diode Charge transfer detector Photoconductor Thermocouple (voltage) or barometer (resistance) Thermal detectors Golay pneumatic cell Pyroelectric cell (capacitance) 30 Chemistry Department, University of Isfahan

31

Radiation Detectors convert light energy to an electrical signal. l In spectroscopy, they are typically placed after a wavelength separator to detect a selected wavelength of light. l Different types of detectors are sensitive in different parts of the electromagnetic spectrum. l 32 Chemistry Department, University of Isfahan

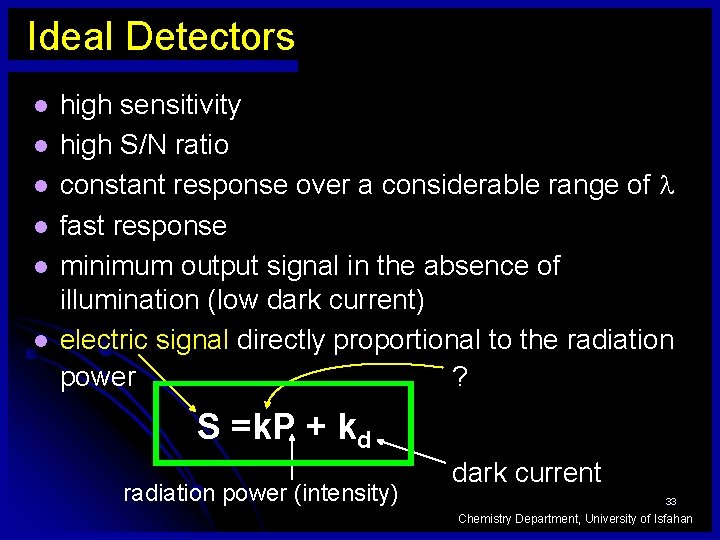
Ideal Detectors l l l high sensitivity high S/N ratio constant response over a considerable range of fast response minimum output signal in the absence of illumination (low dark current) electric signal directly proportional to the radiation ? power S =k. P + kd radiation power (intensity) dark current 33 Chemistry Department, University of Isfahan

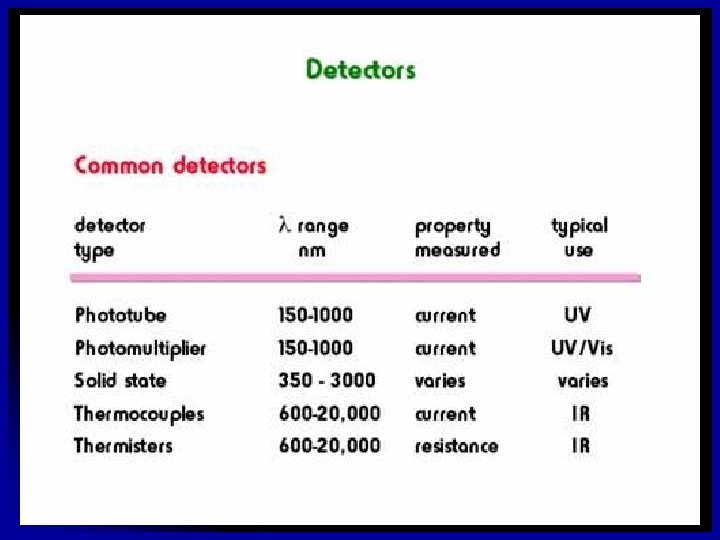
Types of radiation detectors Two major types: one responses to photons, the other to heat l photon detectors: UV, visible, IR used for 3 µm or longer , cooling to dry ice or liquid nitrogen is necessary to avoid interference with thermal signal) l signal results from a series of individual events l shot noise limited l (when l thermal detectors: IR l signal responds to the average power of the incident radiation l thermal noise limited 34 Chemistry Department, University of Isfahan

Photon detectors (a) Photovoltaic cells: radiant energy generates a current at the interface of a semiconductor layer and a metal; (b) Phototubes: radiation causes emission of electrons from a photosensitive solid surface; (c) Phtomultiplier tubes: contain a photoemissive surface as well (d) Photoconductivity detectors: absorption of radiation by a (e) Silicon photodiods: photons increase the conductance as several additional surfaces that emit a cascade of electrons when struck by electrons from the photosensitive area; semiconductor produces electrons and holes, thus leading to enhanced conductivity; across a reverse biased pn junction. Used as diode array to observe the entire spectrum simultaneously (f) Multichannel photon detector 35 Chemistry Department, University of Isfahan

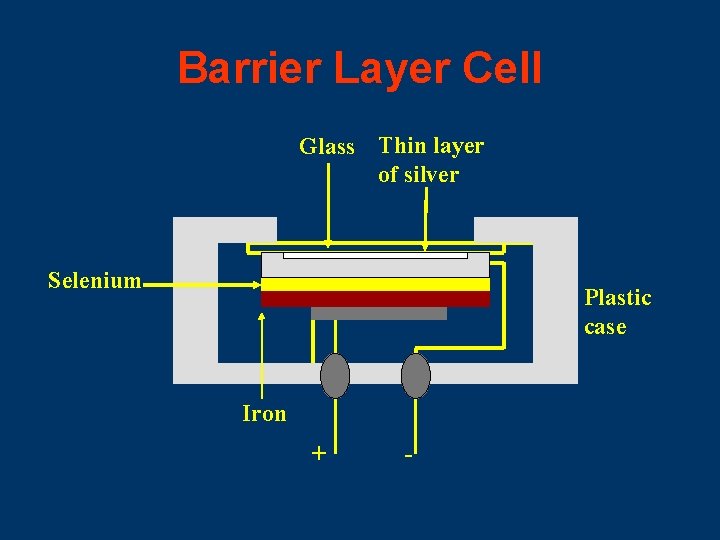
Barrier Layer Cell Glass Thin layer of silver Selenium Plastic case Iron + -

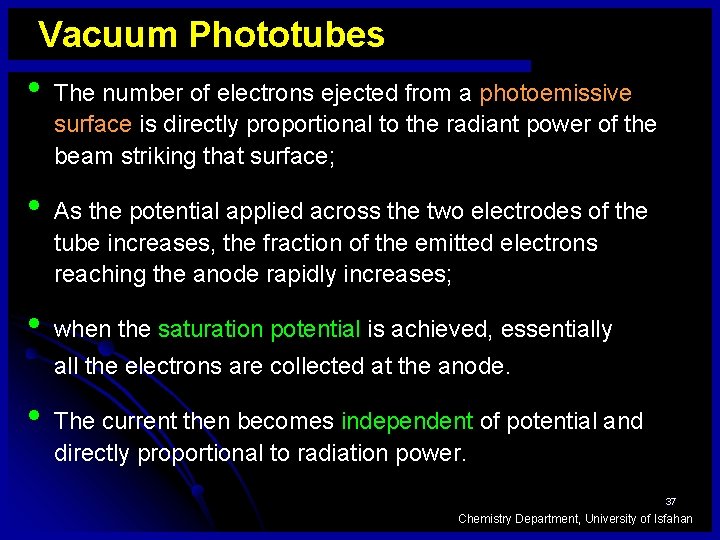
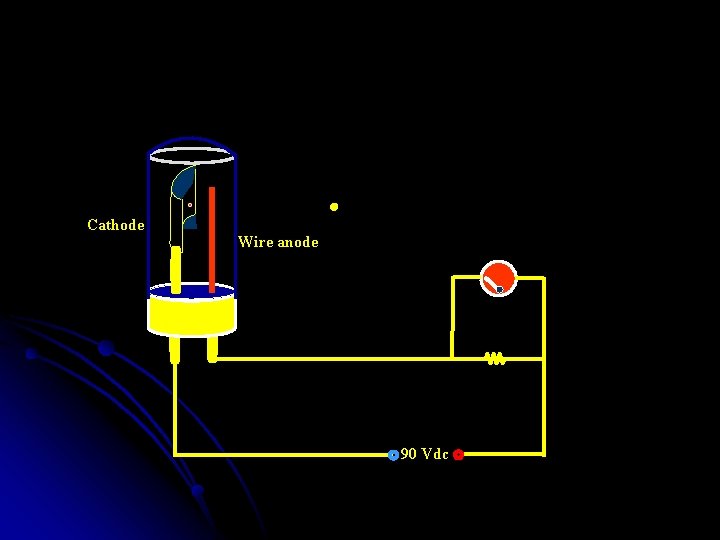
Vacuum Phototubes • • • The number of electrons ejected from a photoemissive surface is directly proportional to the radiant power of the beam striking that surface; As the potential applied across the two electrodes of the tube increases, the fraction of the emitted electrons reaching the anode rapidly increases; when the saturation potential is achieved, essentially all the electrons are collected at the anode. • The current then becomes independent of potential and directly proportional to radiation power. 37 Chemistry Department, University of Isfahan

Cathode Wire anode 90 Vdc

Photomultiplier Tube (PMT) l Photomultiplier Tubes (PMTS) are light detectors that are useful in low intensity applications such as fluorescence spectroscopy. l Due to high internal gain, PMTs are very sensitive detectors. l PMTs are similar to phototubes. They consist of a photocathode and a series of dynodes in an evacuated glass enclosure. l Photons that strikes the photoemissive cathode emits electrons due to the photoelectric effect. 39 Chemistry Department, University of Isfahan

Photomultiplier Tube (PMT) l Instead of collecting these few electrons at an anode like in the phototubes, the electrons are accelerated towards a series of additional electrodes called dynodes. l These electrodes are each maintained at a more positive potential. l Additional electrons are generated at each dynode. 40 Chemistry Department, University of Isfahan

Photomultiplier Tube (PMT) l This cascading effect creates 105 to 107 electrons for each photon hitting the first cathode depending on the number of dynodes and the accelerating voltage. l This amplified signal is finally collected at the anode where it can be measured. 41 Chemistry Department, University of Isfahan

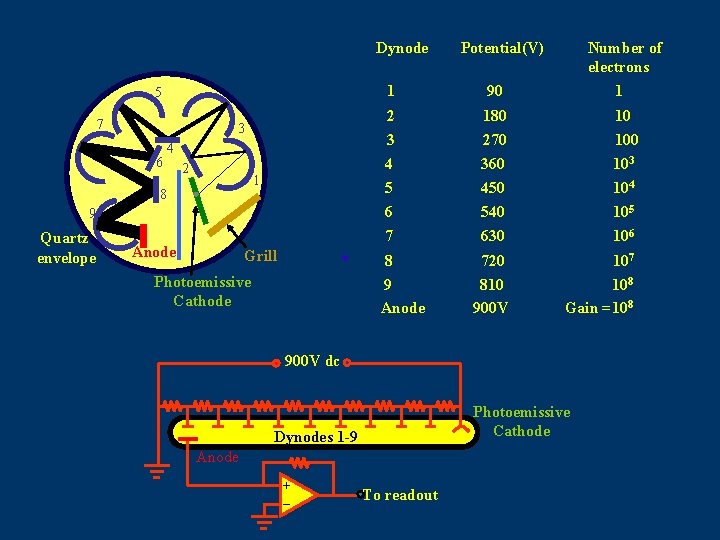
Dynode Potential(V) Number of electrons 1 90 1 2 180 10 3 270 100 4 360 103 5 450 104 9 6 540 105 Quartz envelope 7 630 106 8 720 107 810 900 V 108 Gain =108 5 7 3 6 4 2 1 8 Anode Grill Photoemissive Cathode 9 Anode 900 V dc Photoemissive Cathode Dynodes 1 -9 Anode + _ To readout