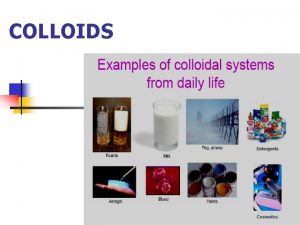
Dispersed systems The methods of preparing of colloidal















- Slides: 32

Dispersed systems. The methods of preparing of colloidal solutions. Ph. D Halina Falfushynska

Dispersed Systems A kinetically stable mixture of one phase in another largely immiscible phase. Usually at least one length scale is in the colloidal range.

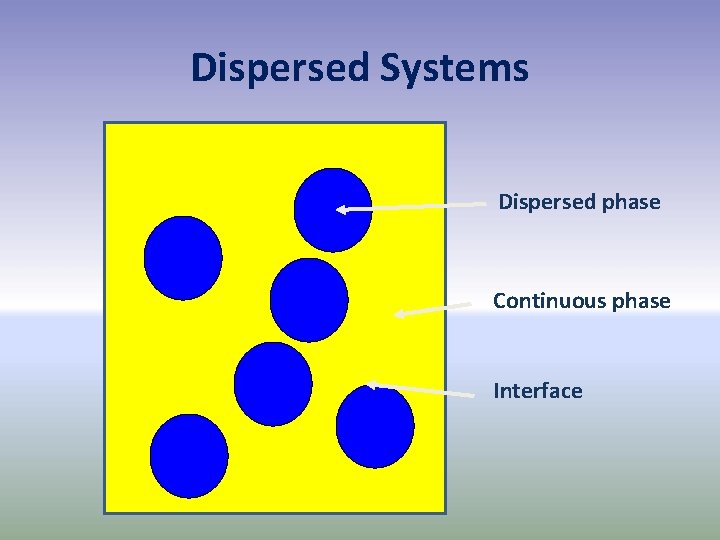
Dispersed Systems Dispersed phase Continuous phase Interface

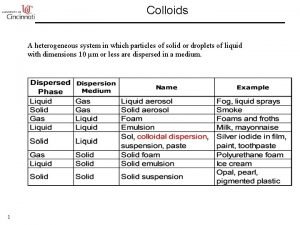
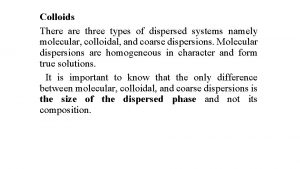
Classification of dispersed system in agreement with particles scale Molecular dispersions is a true solutions of a solute phase in a solvent. The dispersed phase (solute) is in form of separate molecules homogeneously distributed throughout the dispersion medium(solvent). The molecule size is less than 1 nm (4*10 -8 inch). [The examples : air (a molecular mixture of Oxygen, Nitrogen and some other gases), electrolytes (aqueous solutions of salts)].

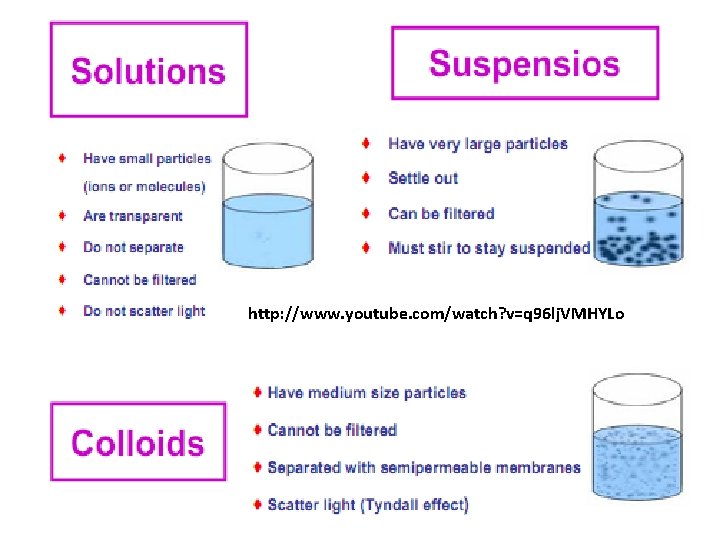
Classification of dispersed system in agreement with particles scale Colloids are micro-heterogeneous dispersed systems, in which the size of the dispersed phase particles is within the range 1 - 1000 nm (4*10 -8 4*10 -5 inch). The colloids phases can not be separated under gravity, centrifugal or other forces. Dispersed phase of colloids may be separated from the dispersion medium by micro-filtration. The examples of colloids: milk (emulsion of fat and some other substances in water), fog (aerosol of water micro-droplets in air), opal (colloidal silica), Silica aerogel monolith, Alumina aerogel monolith].

Classification of dispersed system in agreement with particles scale Coarse dispersions (suspensions) are heterogeneous dispersed systems, in which the dispersed phase particles are larger than 1000 nm (4*10 -5“). Coarse dispersions are characterized by relatively fast sedimentation of the dispersed phase caused by gravity or other forces. Dispersed phase of coarse dispersions may be easily separated from the continuous phase by filtration.


http: //www. youtube. com/watch? v=q 96 lj. VMHYLo



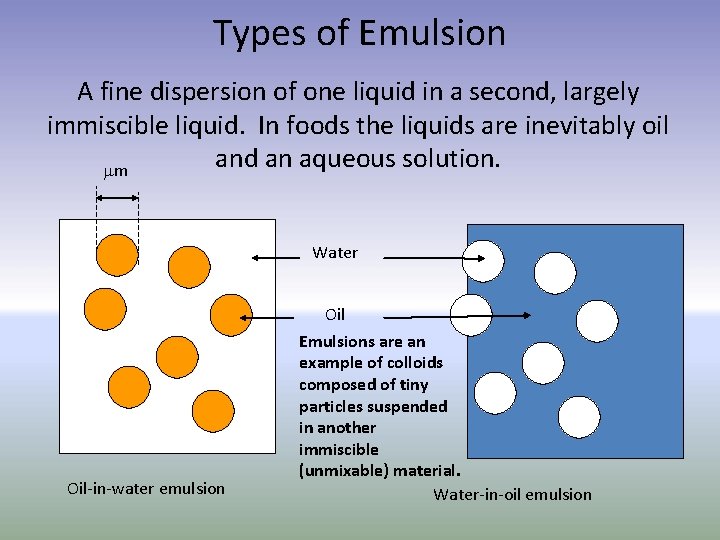
Types of Emulsion A fine dispersion of one liquid in a second, largely immiscible liquid. In foods the liquids are inevitably oil and an aqueous solution. mm Water Oil-in-water emulsion Oil Emulsions are an example of colloids composed of tiny particles suspended in another immiscible (unmixable) material. Water-in-oil emulsion


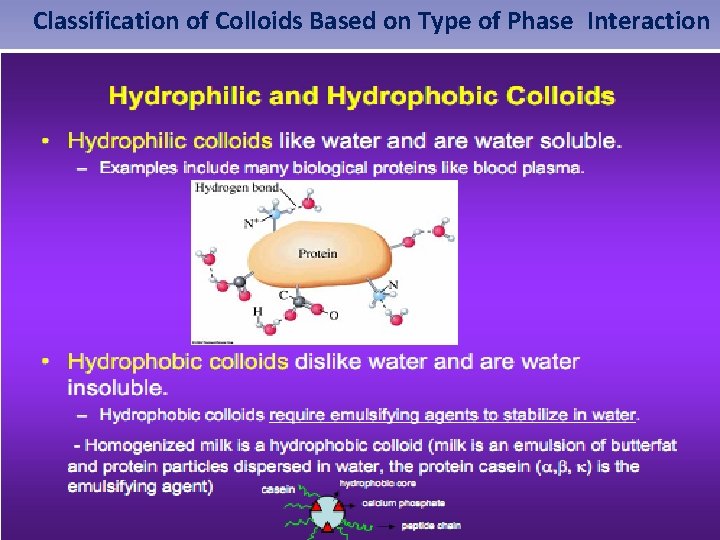
Classification of Colloids Based on Type of Phase Interaction


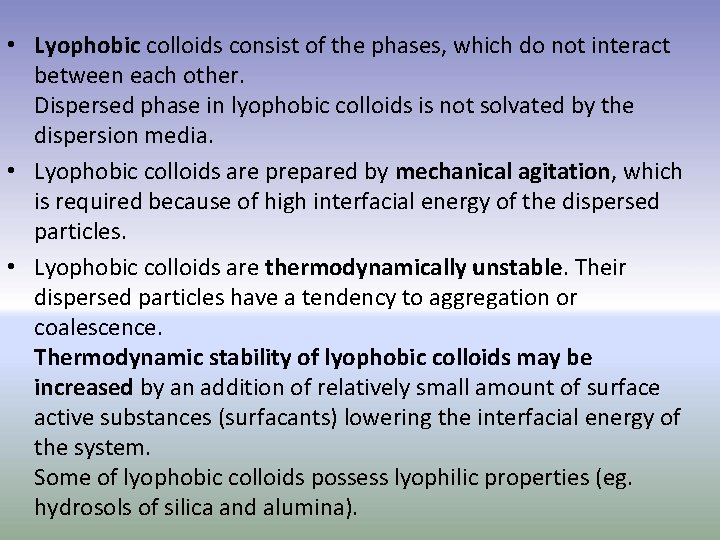
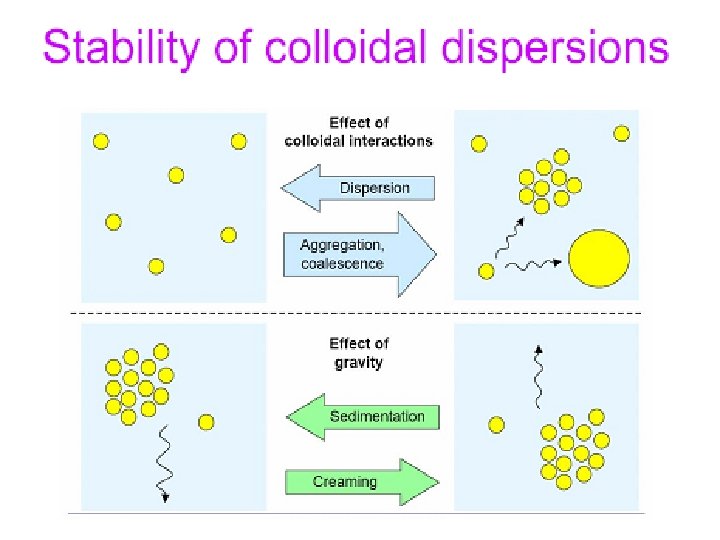
• Lyophobic colloids consist of the phases, which do not interact between each other. Dispersed phase in lyophobic colloids is not solvated by the dispersion media. • Lyophobic colloids are prepared by mechanical agitation, which is required because of high interfacial energy of the dispersed particles. • Lyophobic colloids are thermodynamically unstable. Their dispersed particles have a tendency to aggregation or coalescence. Thermodynamic stability of lyophobic colloids may be increased by an addition of relatively small amount of surface active substances (surfacants) lowering the interfacial energy of the system. Some of lyophobic colloids possess lyophilic properties (eg. hydrosols of silica and alumina).

http: //www. youtube. com/watch? v=-j. Zyqq. N 4 uqc&feature=related

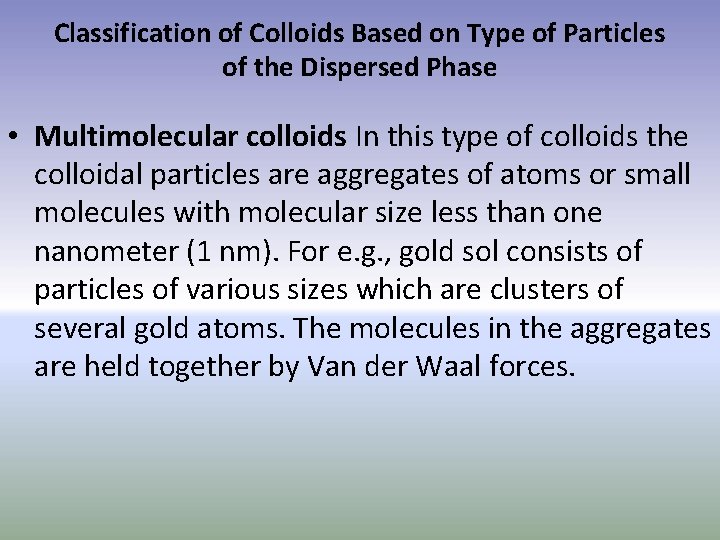
Classification of Colloids Based on Type of Particles of the Dispersed Phase • Multimolecular colloids In this type of colloids the colloidal particles are aggregates of atoms or small molecules with molecular size less than one nanometer (1 nm). For e. g. , gold sol consists of particles of various sizes which are clusters of several gold atoms. The molecules in the aggregates are held together by Van der Waal forces.

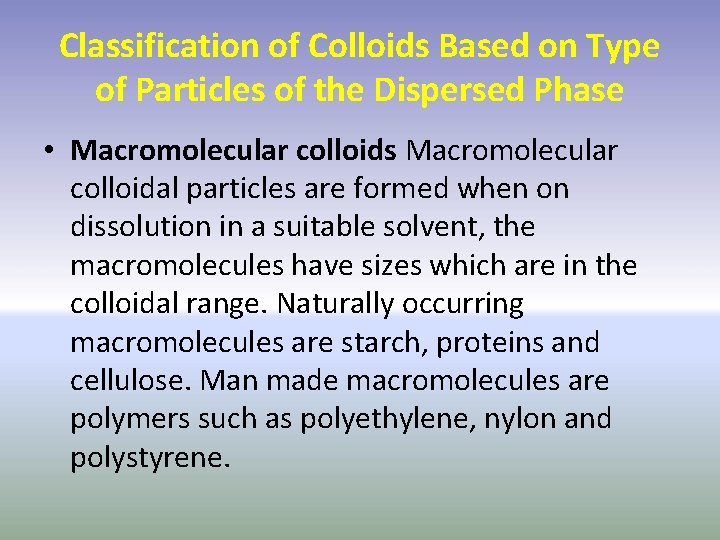
Classification of Colloids Based on Type of Particles of the Dispersed Phase • Macromolecular colloids Macromolecular colloidal particles are formed when on dissolution in a suitable solvent, the macromolecules have sizes which are in the colloidal range. Naturally occurring macromolecules are starch, proteins and cellulose. Man made macromolecules are polymers such as polyethylene, nylon and polystyrene.

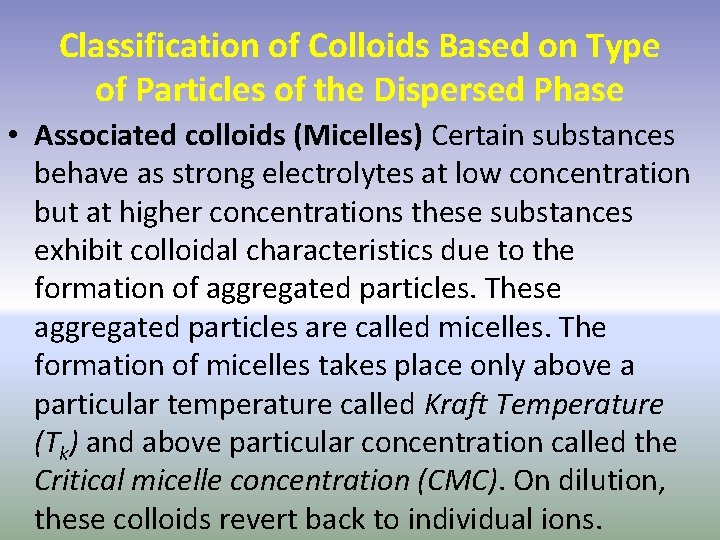
Classification of Colloids Based on Type of Particles of the Dispersed Phase • Associated colloids (Micelles) Certain substances behave as strong electrolytes at low concentration but at higher concentrations these substances exhibit colloidal characteristics due to the formation of aggregated particles. These aggregated particles are called micelles. The formation of micelles takes place only above a particular temperature called Kraft Temperature (Tk) and above particular concentration called the Critical micelle concentration (CMC). On dilution, these colloids revert back to individual ions.

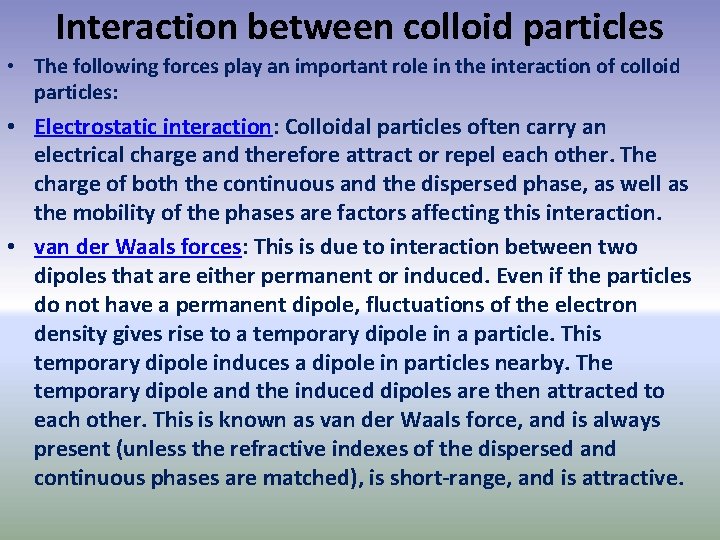
Interaction between colloid particles • The following forces play an important role in the interaction of colloid particles: • Electrostatic interaction: Colloidal particles often carry an electrical charge and therefore attract or repel each other. The charge of both the continuous and the dispersed phase, as well as the mobility of the phases are factors affecting this interaction. • van der Waals forces: This is due to interaction between two dipoles that are either permanent or induced. Even if the particles do not have a permanent dipole, fluctuations of the electron density gives rise to a temporary dipole in a particle. This temporary dipole induces a dipole in particles nearby. The temporary dipole and the induced dipoles are then attracted to each other. This is known as van der Waals force, and is always present (unless the refractive indexes of the dispersed and continuous phases are matched), is short-range, and is attractive.

Interaction between colloid particles • Entropic forces: According to the second law of thermodynamics, a system progresses to a state in which entropy is maximized. This can result in effective forces even between hard spheres. • Steric forces between polymer-covered surfaces or in solutions containing non-adsorbing polymer can modulate interparticle forces, producing an additional steric repulsive force (which is predominantly entropic in origin) or an attractive depletion force between them.


Preparation of Lyophilic Sols • Since lyophilic sols are quite stable, they can be easily prepared by shaking the lyophilic substance with the dispersion medium. • Examples are: Colloidal sols of gum, starch, gelatine and egg albumin.

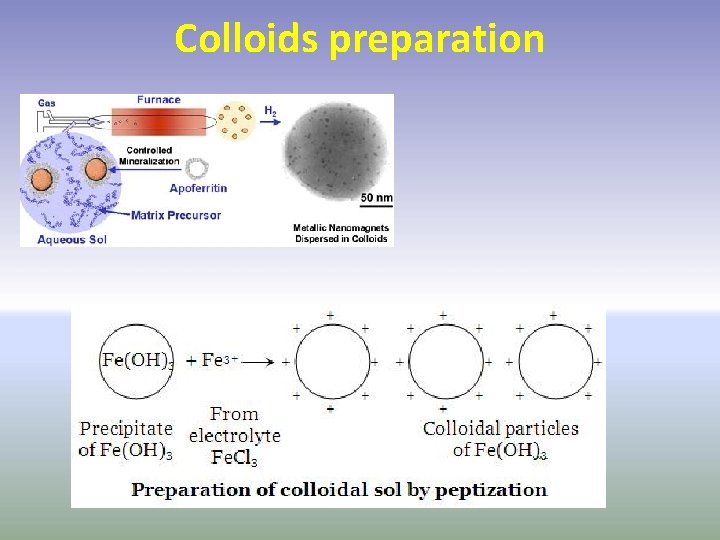
Preparation of Lyophobic Sols • Lyophobic sols are prepared by two methods. They are: • 1) Condensation methods - In condensation methods particles of atomic or molecular size are induced to combine to form aggregates of colloidal dimensions. To achieve this, chemical as well as physical methods are employed. • 2) Dispersion methods. - In dispersion methods, colloidal particles are obtained by breaking large particles of a substance in the presence of a dispersion medium. Since the sols formed are unstable, they are stabilized by adding stabilizing agents.

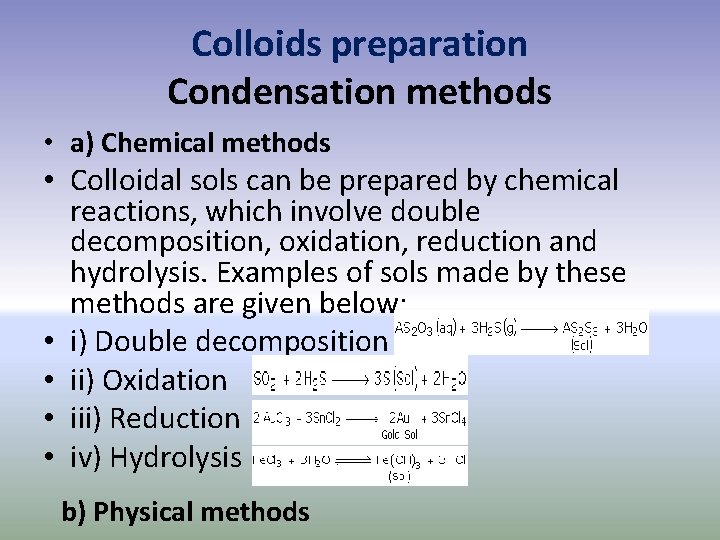
Colloids preparation Condensation methods • a) Chemical methods • Colloidal sols can be prepared by chemical reactions, which involve double decomposition, oxidation, reduction and hydrolysis. Examples of sols made by these methods are given below: • i) Double decomposition • ii) Oxidation • iii) Reduction • iv) Hydrolysis b) Physical methods

Colloids preparation

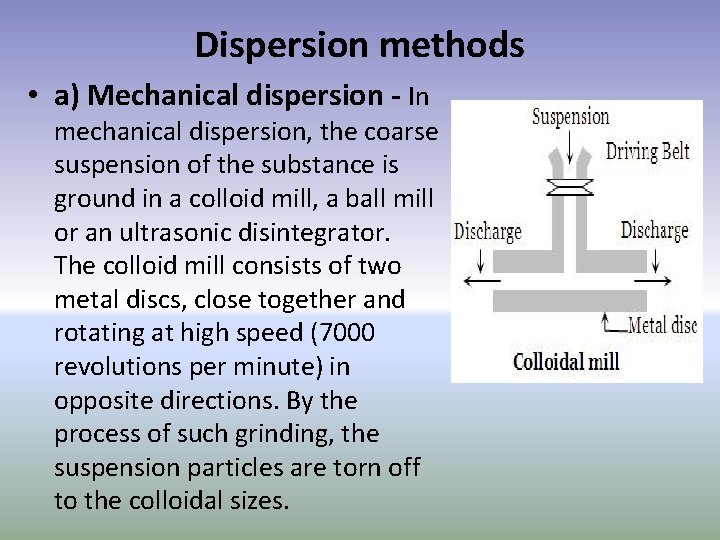
Dispersion methods • a) Mechanical dispersion - In mechanical dispersion, the coarse suspension of the substance is ground in a colloid mill, a ball mill or an ultrasonic disintegrator. The colloid mill consists of two metal discs, close together and rotating at high speed (7000 revolutions per minute) in opposite directions. By the process of such grinding, the suspension particles are torn off to the colloidal sizes.

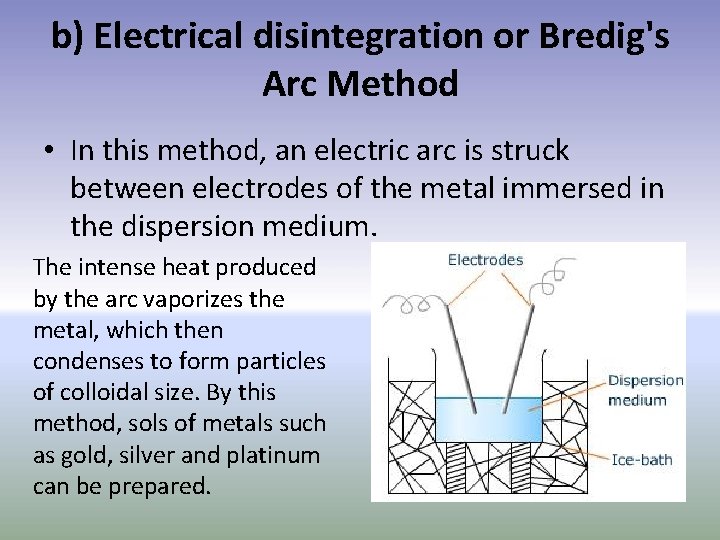
b) Electrical disintegration or Bredig's Arc Method • In this method, an electric arc is struck between electrodes of the metal immersed in the dispersion medium. The intense heat produced by the arc vaporizes the metal, which then condenses to form particles of colloidal size. By this method, sols of metals such as gold, silver and platinum can be prepared.

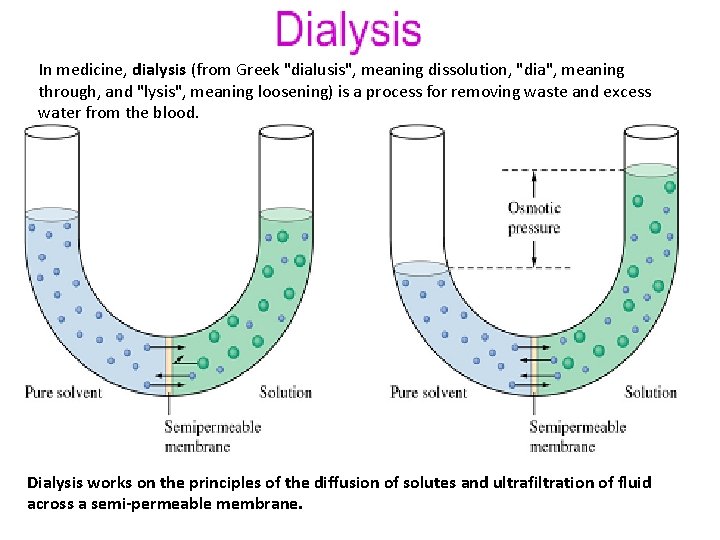
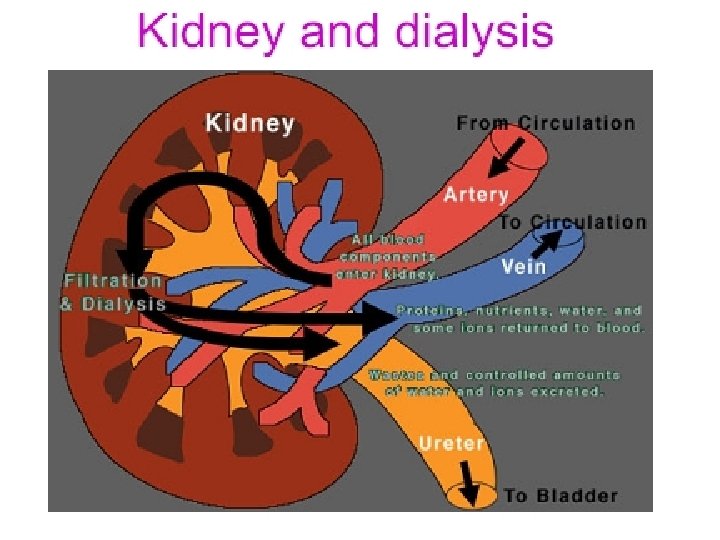
In medicine, dialysis (from Greek "dialusis", meaning dissolution, "dia", meaning through, and "lysis", meaning loosening) is a process for removing waste and excess water from the blood. Dialysis works on the principles of the diffusion of solutes and ultrafiltration of fluid across a semi-permeable membrane.

Dialysis


 Ibm geographically dispersed resiliency for power systems
Ibm geographically dispersed resiliency for power systems Ibm high availability solutions
Ibm high availability solutions Definition of colloid systems in food preparation?
Definition of colloid systems in food preparation? Wet gum method ratio
Wet gum method ratio Examples of decomposition reaction
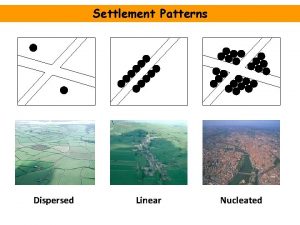
Examples of decomposition reaction Nucleated settlement advantages and disadvantages
Nucleated settlement advantages and disadvantages Good settlement
Good settlement Nucleated settlement pattern
Nucleated settlement pattern Flocculated and deflocculated suspension
Flocculated and deflocculated suspension Dispersion phase and dispersion medium
Dispersion phase and dispersion medium Interfacial properties of suspended particles ppt
Interfacial properties of suspended particles ppt Metes and bounds ap human geography
Metes and bounds ap human geography Seed splitting
Seed splitting Mango dispersal
Mango dispersal Dispersed
Dispersed Geographically dispersed parallel sysplex
Geographically dispersed parallel sysplex Dispersed rural settlement definition
Dispersed rural settlement definition Need a service chapter 12
Need a service chapter 12 Dispersed phase
Dispersed phase Conventional crowd
Conventional crowd Metal coping fpd
Metal coping fpd Which are not purely surface phenomena
Which are not purely surface phenomena What is a sol
What is a sol Colloid is a heterogeneous system
Colloid is a heterogeneous system Definition of colloidal
Definition of colloidal Colloidal silica gun mix
Colloidal silica gun mix Stan jones blue
Stan jones blue Cengage
Cengage Difference between dry and wet calcination
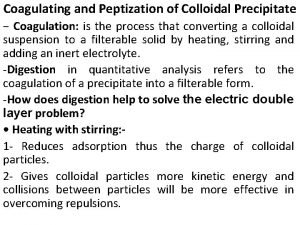
Difference between dry and wet calcination Peptization
Peptization Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Mixed crystal formation in gravimetric analysis
Mixed crystal formation in gravimetric analysis Effect of electrolytes on colloidal dispersion
Effect of electrolytes on colloidal dispersion