Dispersed Systems FDSC 400 2004 Version Goals Scales

















- Slides: 38

Dispersed Systems FDSC 400 2004 Version

Goals • • • Scales and Types of Structure in Food Surface Tension Curved Surfaces Surface Active Materials Charged Surfaces

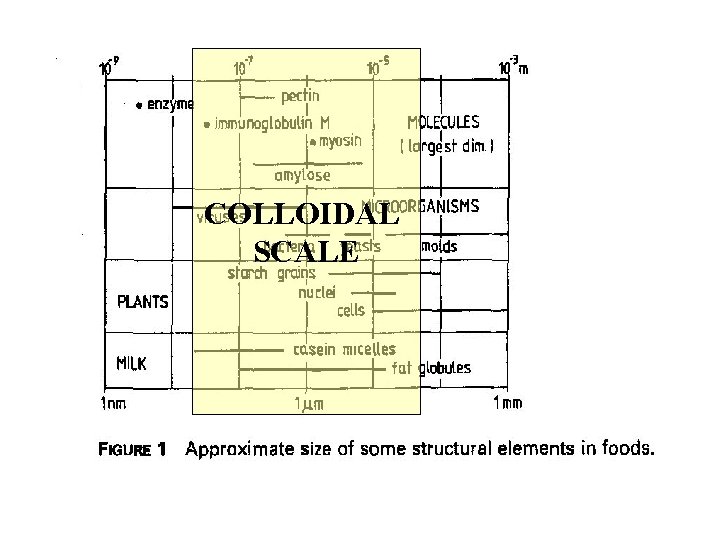
COLLOIDAL SCALE

Dispersed Systems A kinetically stable mixture of one phase in another largely immiscible phase. Usually at least one length scale is in the colloidal range.

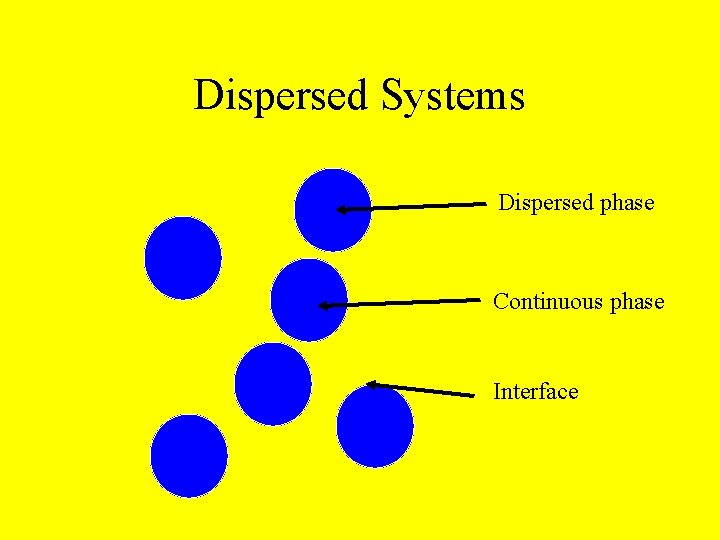
Dispersed Systems Dispersed phase Continuous phase Interface

Dispersed phase Continuous phase Solid Liquid Gas Some glasses Sol Smoke Liquid Gas Emulsion Aerosol Solid foam Foam

Properties of Dispersed Systems • Too small to see • Affected by both gravitational forces and thermal diffusion • Large interfacial area – SURFACE EFFECTS ARE IMPORTANT

Increased Surface Area We have 20 cm 3 of oil in 1 cm radius droplets. Each has a volume of (4/3. p. r 3) 5. 5 cm 3 and a surface area of (4. p. r 2) 12. 5 cm 2. As we need about 3. 6 droplets we would have a total area of 45. 5 cm 2 The same oil is split into 0. 1 cm radius droplets, each has a volume of 0. 004 cm 3 and a surface area 0. 125 cm 2. As we need about 5000 droplets we would have a total area of 625 cm 2

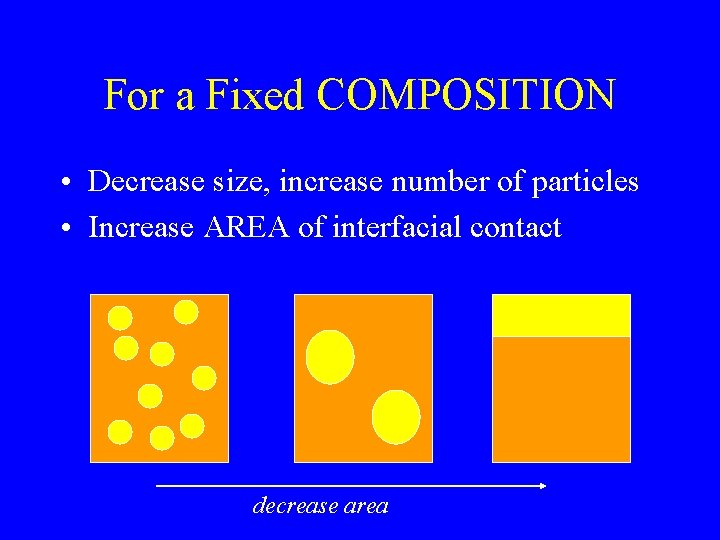
For a Fixed COMPOSITION • Decrease size, increase number of particles • Increase AREA of interfacial contact decrease area

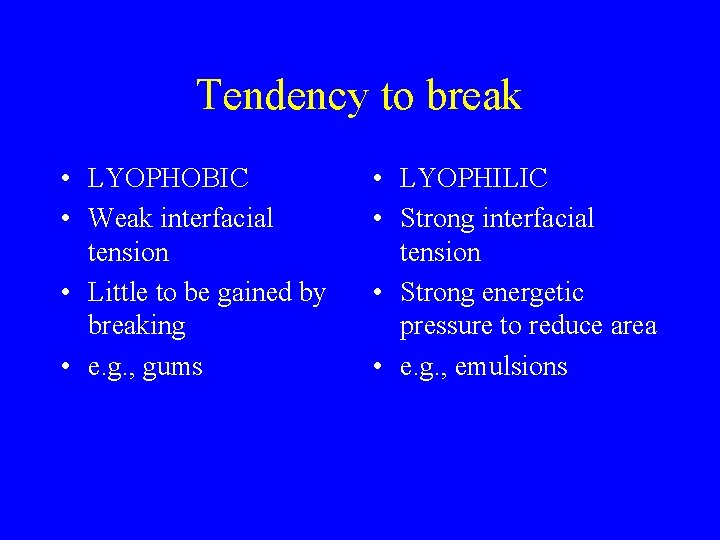
Tendency to break • LYOPHOBIC • Weak interfacial tension • Little to be gained by breaking • e. g. , gums • LYOPHILIC • Strong interfacial tension • Strong energetic pressure to reduce area • e. g. , emulsions

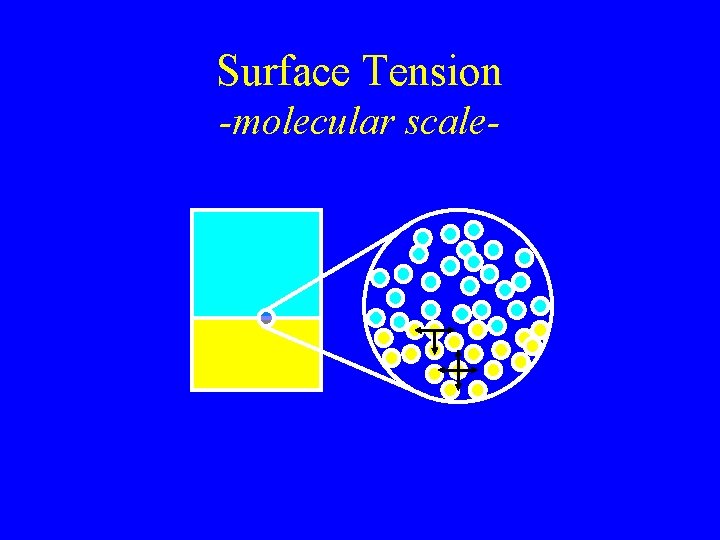
Surface Tension -molecular scale-

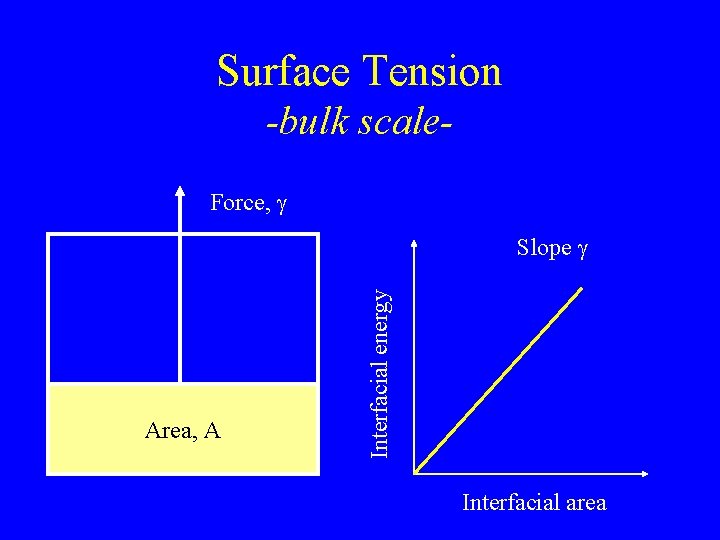
Surface Tension -bulk scale. Force, g Area, A Interfacial energy Slope g Interfacial area

Surface Active Material • Types of surfactant • Surface accumulation • Surface tension lowering

Types of Surfactant -small molecule. Hydrophilic head group (charged or polar) Hydrophobic tail (non-polar)

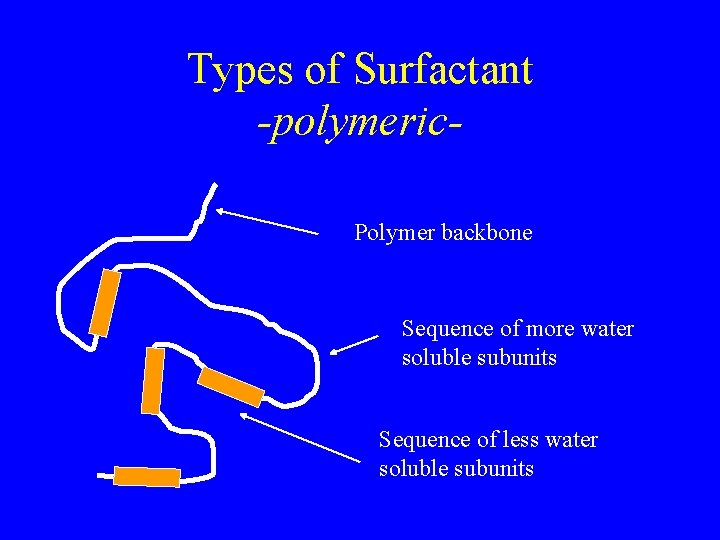
Types of Surfactant -polymeric. Polymer backbone Sequence of more water soluble subunits Sequence of less water soluble subunits

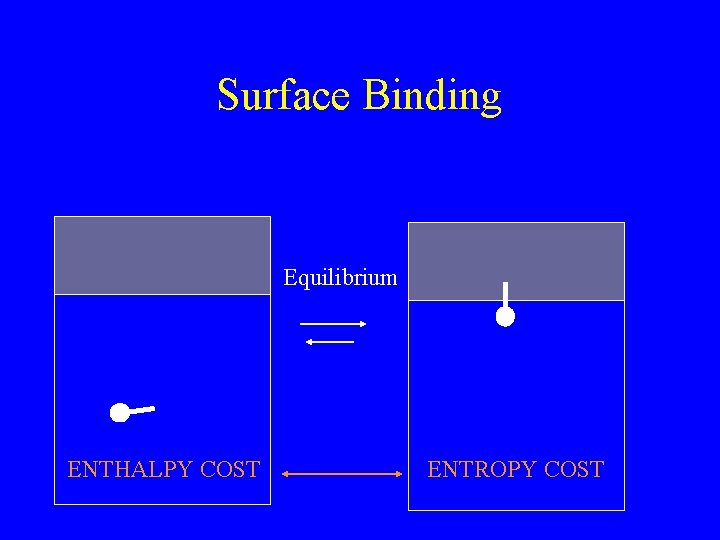
Surface Binding Equilibrium ENTHALPY COST ENTROPY COST

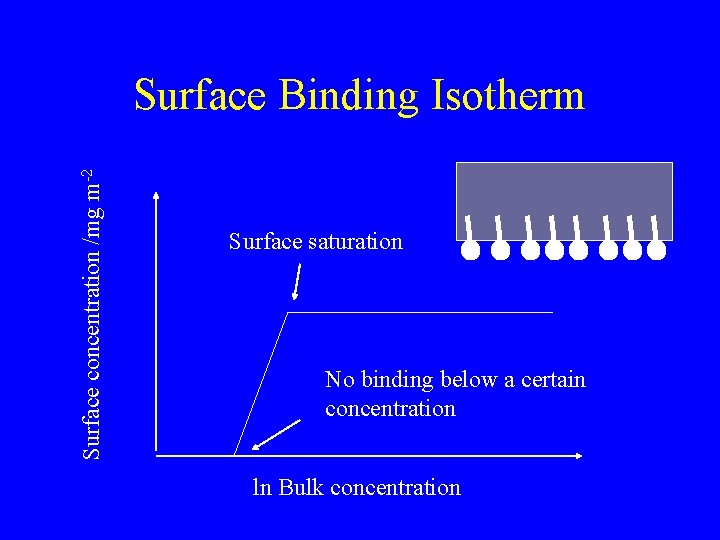
Surface concentration /mg m-2 Surface Binding Isotherm Surface saturation No binding below a certain concentration ln Bulk concentration

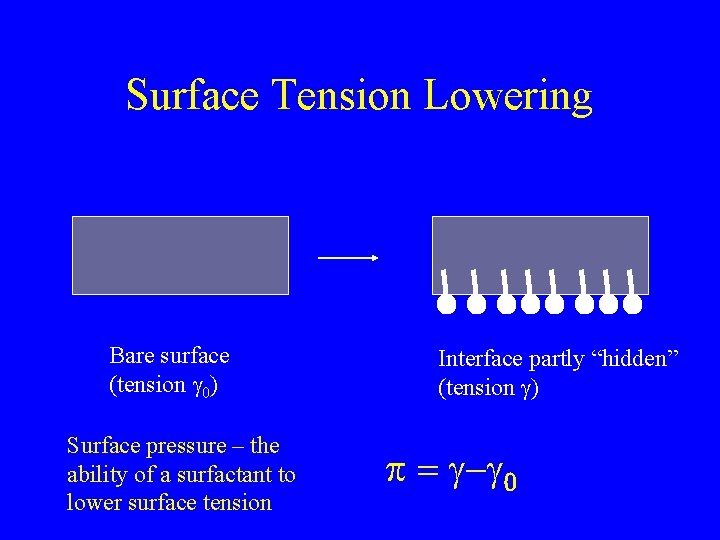
Surface Tension Lowering Bare surface (tension g 0) Surface pressure – the ability of a surfactant to lower surface tension Interface partly “hidden” (tension g) p = g-g 0

Summary • Small particles have a large surface area • Surfaces have energy associated with them (i. e. , they are unstable) because of their interfacial tension • Dispersions will tend to aggregate to reduce the interfacial area • Proteins and small molecule surfactants will adsorb to the surface to reduce surface tension and increase stability.

Example Dispersion: Emulsions

Emulsion A fine dispersion of one liquid in a second, largely immiscible liquid. In foods the liquids are inevitably oil and an aqueous solution.

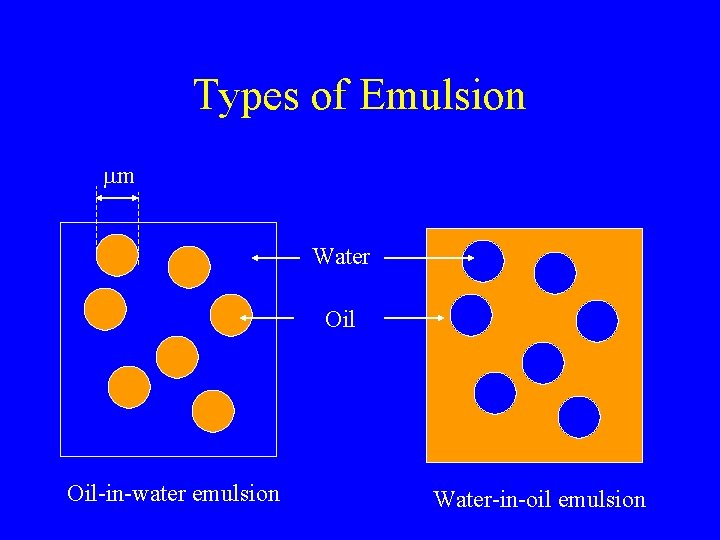
Types of Emulsion mm Water Oil-in-water emulsion Water-in-oil emulsion

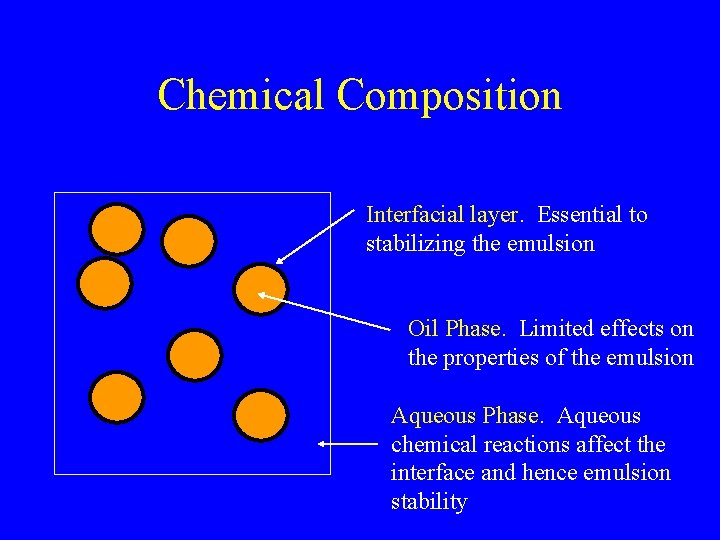
Chemical Composition Interfacial layer. Essential to stabilizing the emulsion Oil Phase. Limited effects on the properties of the emulsion Aqueous Phase. Aqueous chemical reactions affect the interface and hence emulsion stability

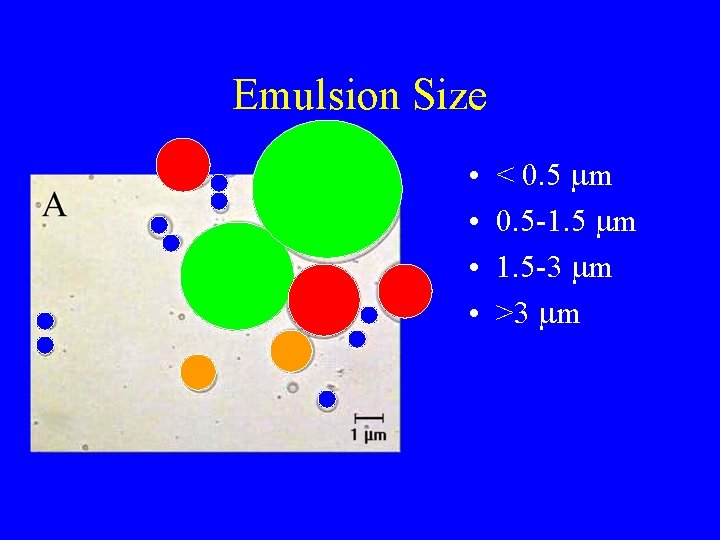
Emulsion Size • • < 0. 5 mm 0. 5 -1. 5 mm 1. 5 -3 mm >3 mm

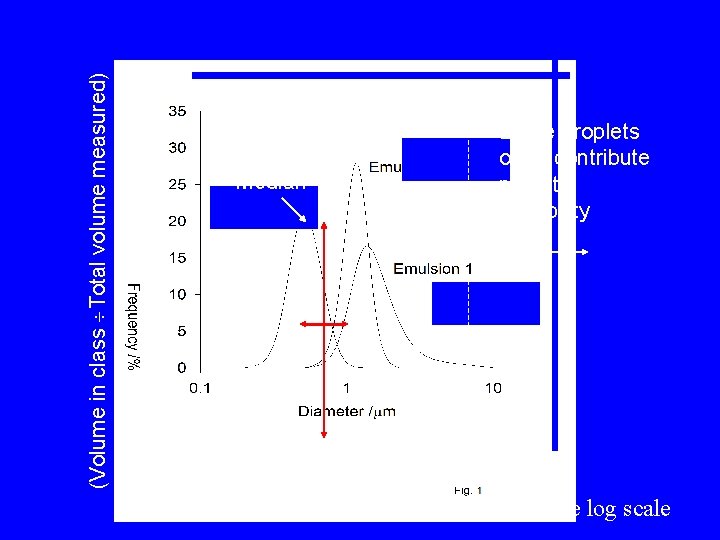
Number Distributions • • < 0. 5 mm 0. 5 -1. 5 mm 1. 5 -3 mm >3 mm Number Very few large droplets contain most of the oil

ty spersi Polydi (Volume in class Total volume measured) Median Large droplets often contribute most to instability Note log scale

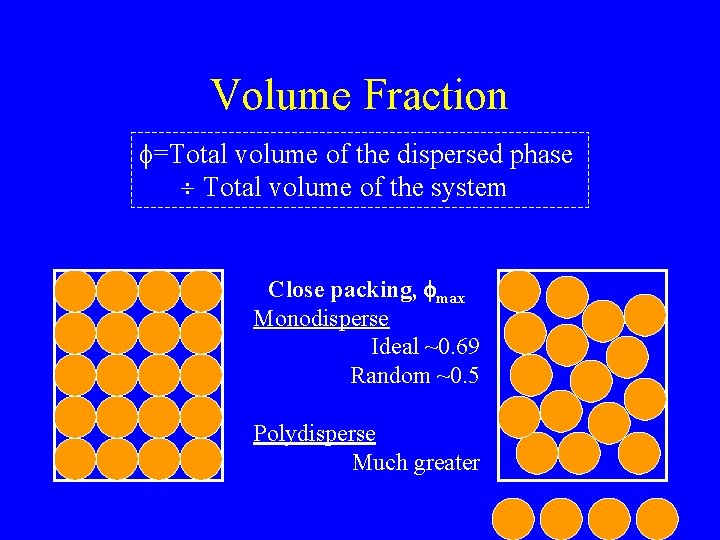
Volume Fraction f=Total volume of the dispersed phase Total volume of the system Close packing, fmax Monodisperse Ideal ~0. 69 Random ~0. 5 Polydisperse Much greater

Emulsion Viscosity of emulsion Continuous phase viscosity Emulsion droplets disrupt streamlines and require more effort to get the same flow rate Dispersed phase volume fraction

Emulsion Destabilization • • Creaming Flocculation Coalescence Combined methods

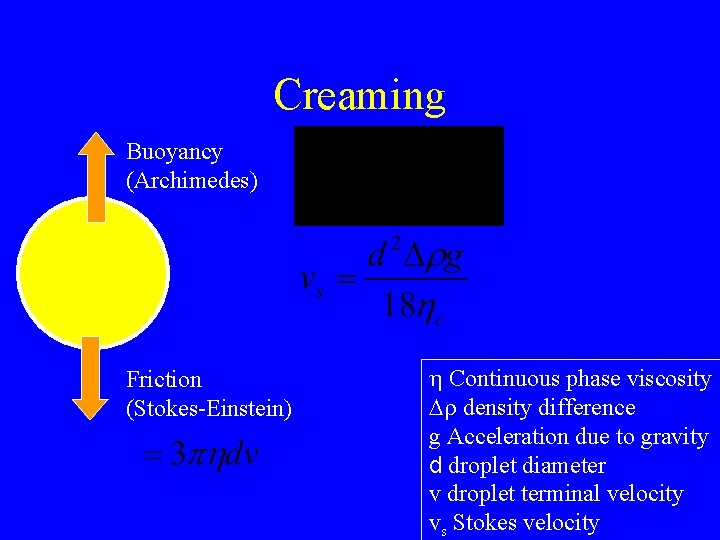
Creaming Buoyancy (Archimedes) Friction (Stokes-Einstein) h Continuous phase viscosity Dr density difference g Acceleration due to gravity d droplet diameter v droplet terminal velocity vs Stokes velocity

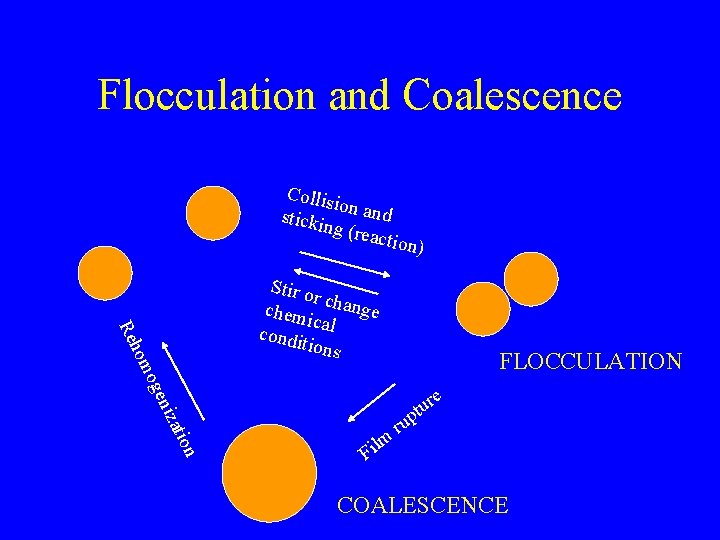
Flocculation and Coalescence Collis io stickin n and g (rea ction) FLOCCULATION atio niz oge hom Re Stir o r chem change ic condi al tions e r u t p n lm i F ru COALESCENCE

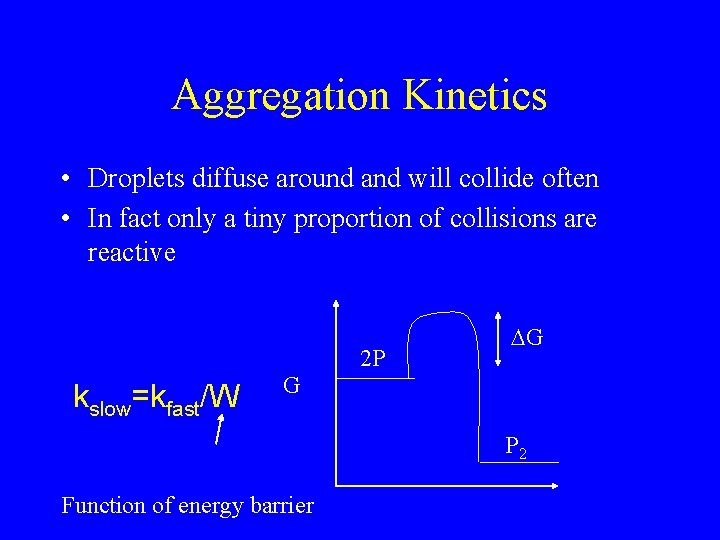
Aggregation Kinetics • Droplets diffuse around and will collide often • In fact only a tiny proportion of collisions are reactive 2 P kslow=kfast/W DG G P 2 Function of energy barrier

Interaction Potential • Non-covalent attractive and repulsive forces will act to pull droplets together (increase flocculation rate) or push them apart (decrease flocculation rate)

Van der Waals Attraction • Always attractive • Very short range

Electrostatic Repulsion • Repulsive or attractive depending on sign of charges • Magnitude depends on magnitude of the charge • Gets weaker with distance but reasonably long range

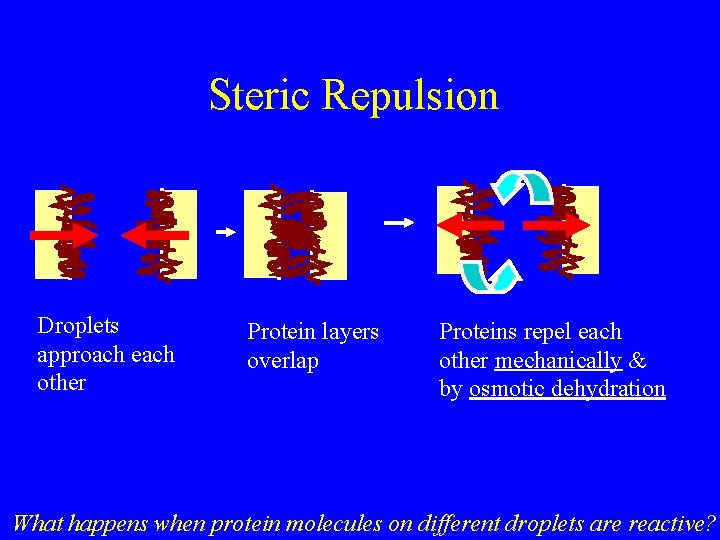
Steric Repulsion Droplets approach each other Protein layers overlap Proteins repel each other mechanically & by osmotic dehydration What happens when protein molecules on different droplets are reactive?

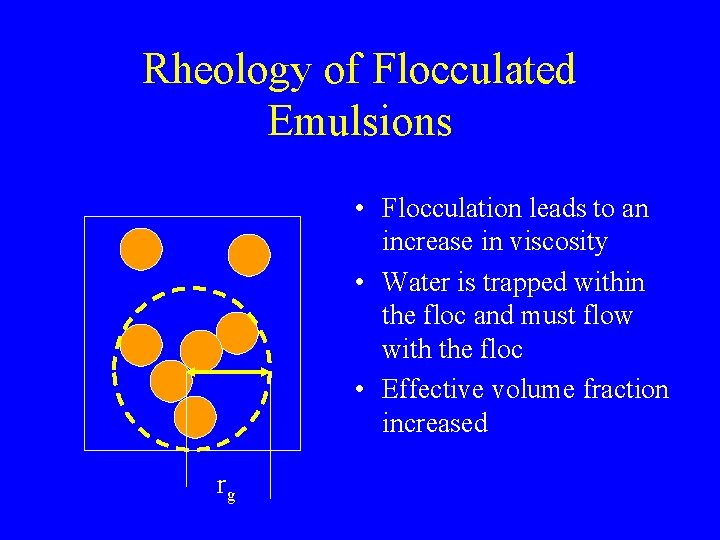
Rheology of Flocculated Emulsions • Flocculation leads to an increase in viscosity • Water is trapped within the floc and must flow with the floc • Effective volume fraction increased rg

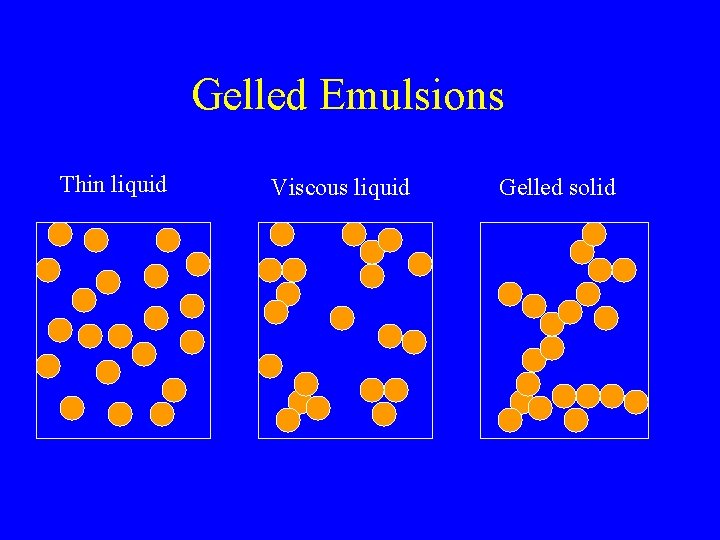
Gelled Emulsions Thin liquid Viscous liquid Gelled solid