Disperse and colloidal systems Jana Novotn Types of










- Slides: 24

Disperse and colloidal systems Jana Novotná

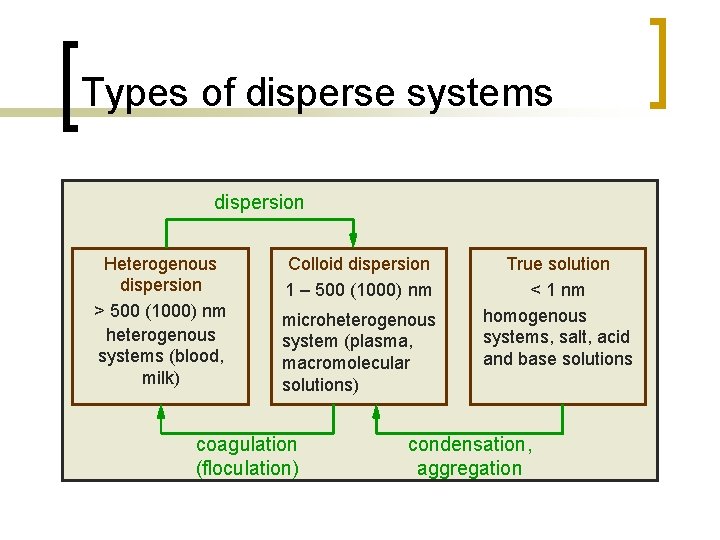
Types of disperse systems n n The term "Disperse System" refers to a system in which one substance (the dispersed phase) is distributed, in discrete units, throughout a second substance (the continuous phase or vehicle). Each phase can exist in solid, liquid, or gaseous state.

Types of disperse systems dispersion Heterogenous dispersion > 500 (1000) nm heterogenous systems (blood, milk) Colloid dispersion 1 – 500 (1000) nm microheterogenous system (plasma, macromolecular solutions) coagulation (floculation) True solution < 1 nm homogenous systems, salt, acid and base solutions condensation, aggregation

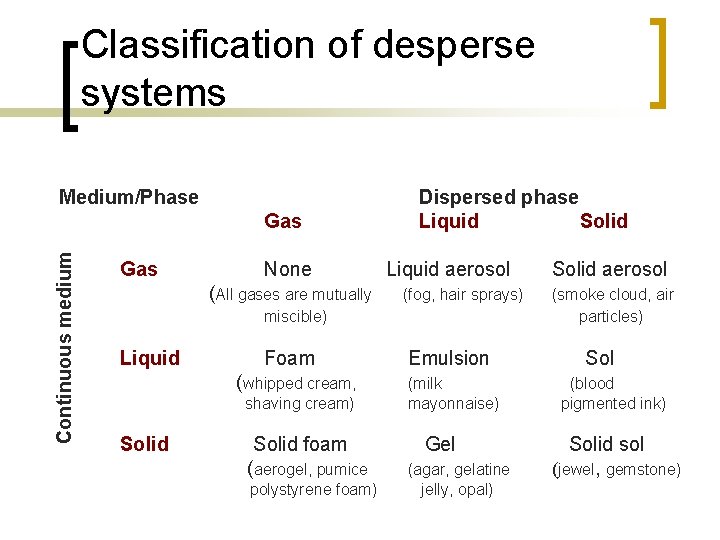
Classification of desperse systems Medium/Phase Continuous medium Gas None (All gases are mutually Dispersed phase Liquid Solid Liquid aerosol (fog, hair sprays) miscible) Liquid Foam (whipped cream, shaving cream) Solid foam (aerogel, pumice polystyrene foam) Emulsion (milk mayonnaise) Gel (agar, gelatine jelly, opal) Solid aerosol (smoke cloud, air particles) Sol (blood pigmented ink) Solid sol (jewel, gemstone)

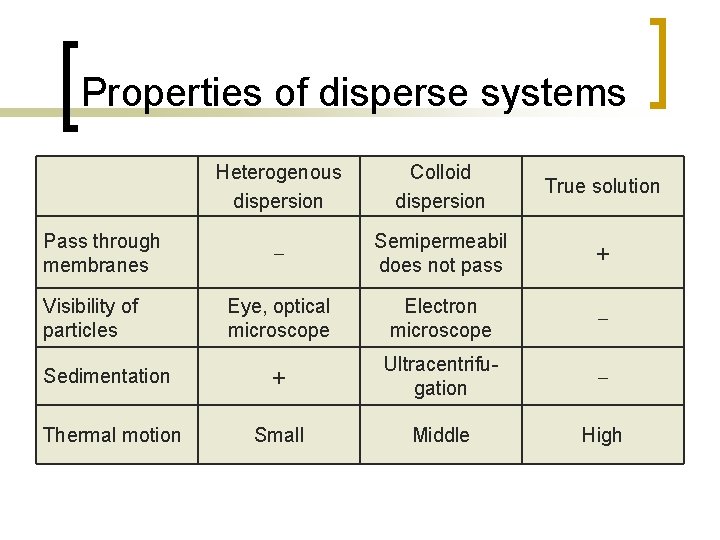
Properties of disperse systems Heterogenous dispersion Colloid dispersion True solution Semipermeabil does not pass + Eye, optical microscope Electron microscope Sedimentation + Ultracentrifugation Thermal motion Small Middle High Pass through membranes Visibility of particles

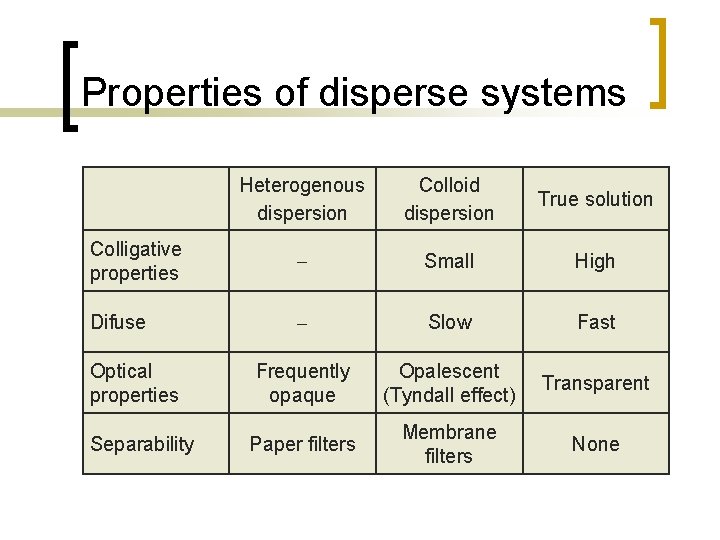
Properties of disperse systems Heterogenous dispersion Colloid dispersion True solution Colligative properties Small High Difuse Slow Fast Frequently opaque Opalescent (Tyndall effect) Transparent Paper filters Membrane filters None Optical properties Separability

Heterogenous (rough) dispersion n n Suspension - heterogeneous fluid containing solid particles that are sufficiently large for sedimentation. Particle size is > 1 mm Dispersion is made by mechanical agitation (sand in the water). Aerosol - a suspension of liquid droplets or a suspension of fine solid particles in a gas. ¡ Example : smoke, air pollution, smog etc.

Heterogenous (rough) dispersion n Emulsion - a mixture of two or more immiscible liquids one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Prepared by shaking – oil/water (milk), water/ oil (butter).

Colloids n n Particle size 1 – 1000 nm. Particles have very large surface area Homogenous colloidal system - lyophilic dispersion. Heterogenic colloidal system – lyophobic dispersion

Properties of colloid solutions n n Particles are visible only by ultramicroskop or electron microskop – Brown motion. They do not sedimentate, pass through common filters ( but not through semipermeable membrane). Dispersion of passing light (Tyndall efect). Produce osmotic pressure.

Properties of colloid solutions n Colloids are everywhere ¡ ¡ In the human body Washing powder, soup, tooth paste, etc. Many foods ( yogurt, butter, milk) Nanotechnologies are based on chemistry of colloids

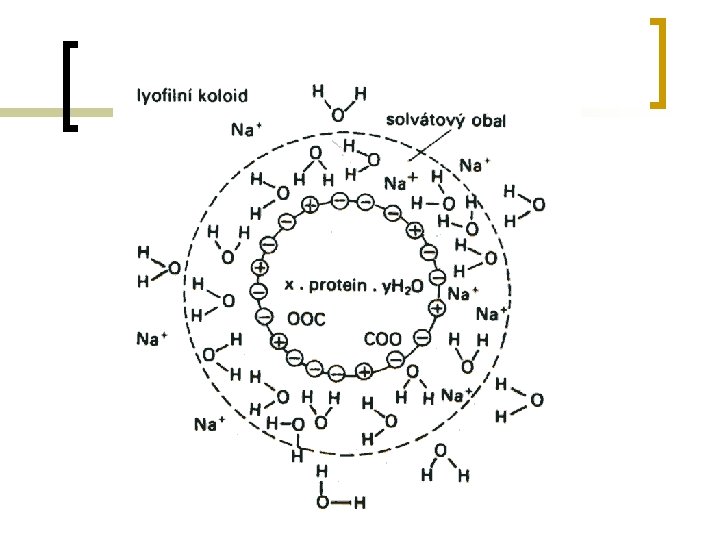
Lyophilic colloids n Lyophilic particles are mixed with the suitable solvent. Particles have high affinity to the solvent. ¡ ¡ ¡ n High force of attraction exists between colloidal particles and liquid. This result in formation of very stable solution called lyophilic sol. Attraction forces (solvate cover) stabilize lyophilic colloid, defense their clustering to the larger complexes Cells – contain solution of lyophilic colloids


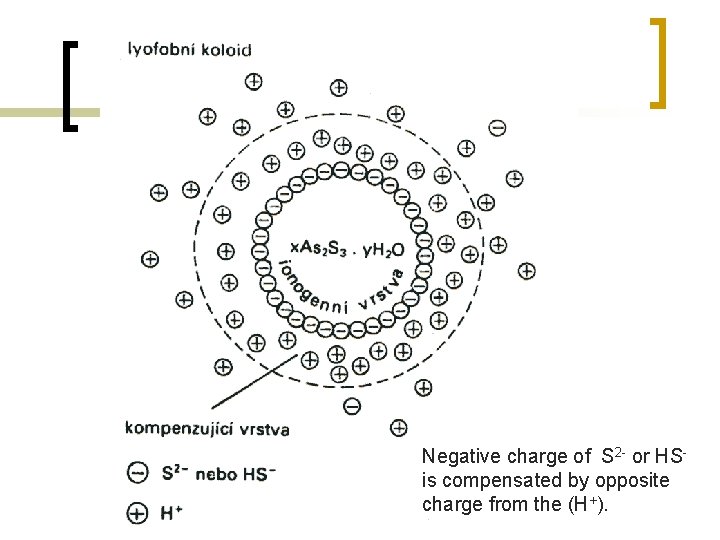
Lyophobic colloid n n n Colloid particles do not have affinity to the molecules of solvent Mostly complexes of inorganic particles which do not have affinity to the solvent They are prepared by artificial dispersion - Fe(OH)3, As 2 S 3

Negative charge of S 2 - or HSis compensated by opposite charge from the (H+).

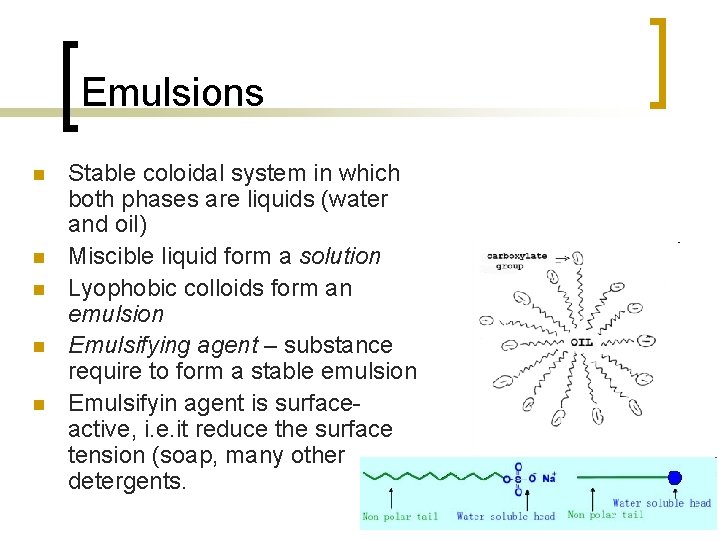
Emulsions n n n Stable coloidal system in which both phases are liquids (water and oil) Miscible liquid form a solution Lyophobic colloids form an emulsion Emulsifying agent – substance require to form a stable emulsion Emulsifyin agent is surfaceactive, i. e. it reduce the surface tension (soap, many other detergents.

Solutions n n n A solution is a homogenous mixture composed of two or more substances. A solute is dissolved in another substance, known as a solvent. Types of solutions: ¡ ¡ Solvent is gas (mixture of different gases) Solvent is liquid n n n ¡ Gas in liquid (water solution of HCl) Liquid in liquid (H 2 SO 4 in the water) Solid in liquid (glucose , Na. Cl in the water) Solvent is solid – alloys like bronze (Cu and Sn)

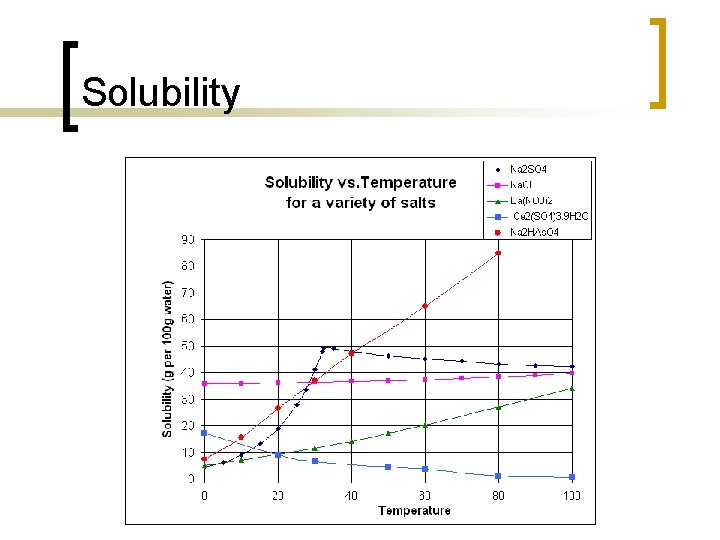
Solubility n n The ability of one compound to dissolve in another compound Miscible liquids - liquid is able to completely dissolve in another liquid (ethanol, water). The solubility of a given solute in a given solvent typically depends on temperature Solubility of ionic compounds - there is a limit to how much salt can be dissolved in a given volume of water

Solubility

Dissolving substances in water n No-electrolytes do not interact with the solvent (oxygen, sucrose). n Electrolytes interact with molecules of the solvent → dissociate, ionize ¡ ¡ The ions are then surrounded by solvent molecules (H 2 O). Hydrated ions then arise.

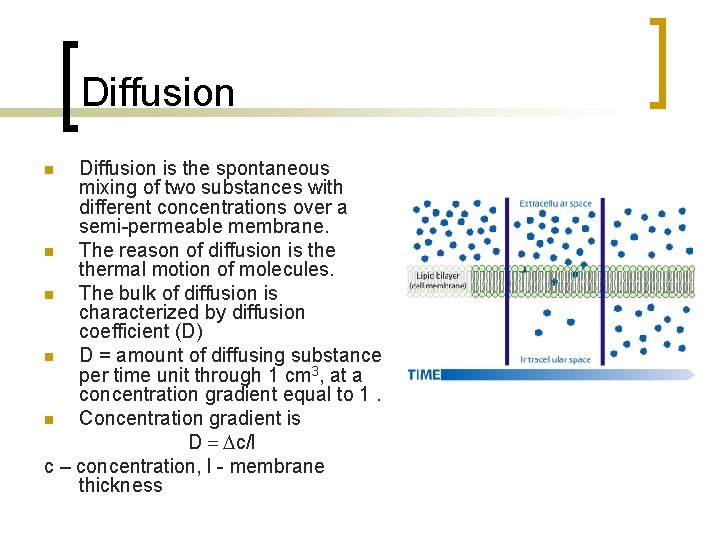
Diffusion is the spontaneous mixing of two substances with different concentrations over a semi-permeable membrane. n The reason of diffusion is thermal motion of molecules. n The bulk of diffusion is characterized by diffusion coefficient (D) n D = amount of diffusing substance per time unit through 1 cm 3, at a concentration gradient equal to 1. n Concentration gradient is D = Dc/l c – concentration, l - membrane thickness n

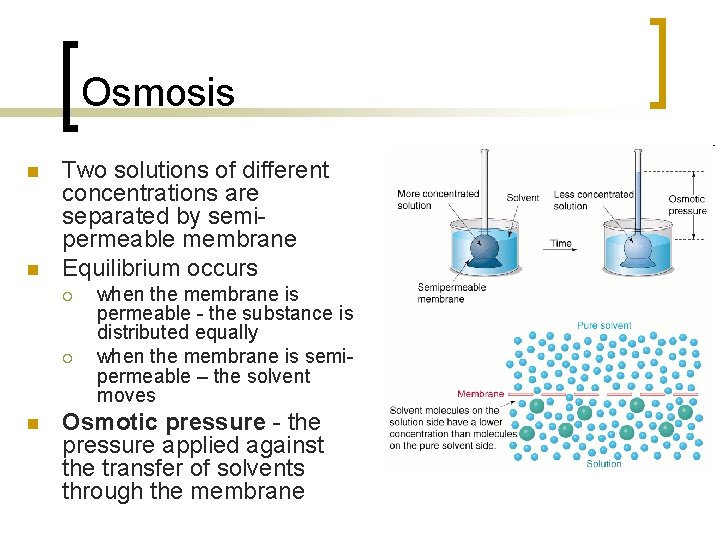
Osmosis n n Two solutions of different concentrations are separated by semipermeable membrane Equilibrium occurs ¡ ¡ n when the membrane is permeable - the substance is distributed equally when the membrane is semipermeable – the solvent moves Osmotic pressure - the pressure applied against the transfer of solvents through the membrane

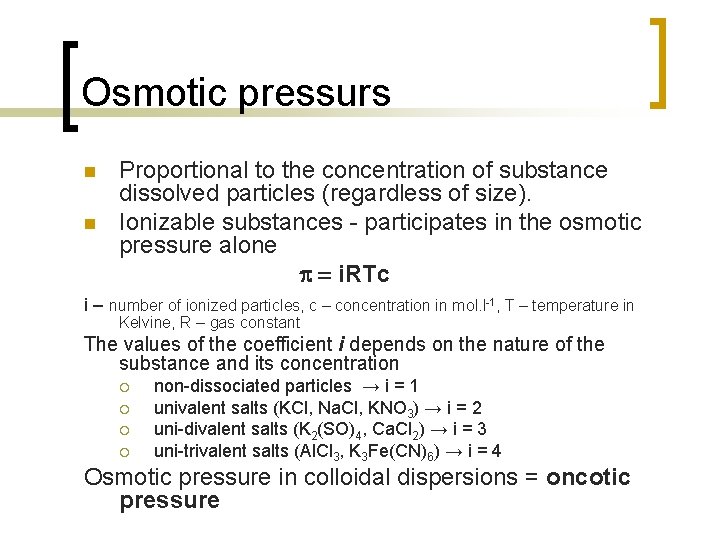
Osmotic pressurs n n Proportional to the concentration of substance dissolved particles (regardless of size). Ionizable substances - participates in the osmotic pressure alone p = i. RTc i – number of ionized particles, c – concentration in mol. l-1, T – temperature in Kelvine, R – gas constant The values of the coefficient i depends on the nature of the substance and its concentration ¡ ¡ non-dissociated particles → i = 1 univalent salts (KCl, Na. Cl, KNO 3) → i = 2 uni-divalent salts (K 2(SO)4, Ca. Cl 2) → i = 3 uni-trivalent salts (Al. Cl 3, K 3 Fe(CN)6) → i = 4 Osmotic pressure in colloidal dispersions = oncotic pressure

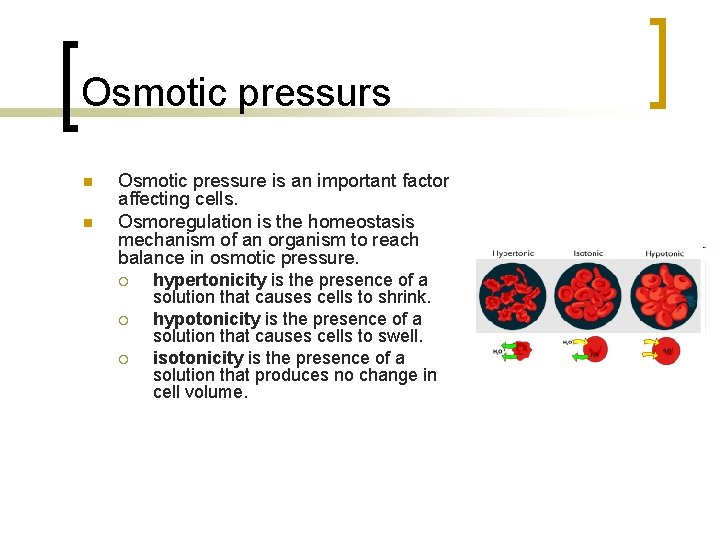
Osmotic pressurs n n Osmotic pressure is an important factor affecting cells. Osmoregulation is the homeostasis mechanism of an organism to reach balance in osmotic pressure. ¡ ¡ ¡ hypertonicity is the presence of a solution that causes cells to shrink. hypotonicity is the presence of a solution that causes cells to swell. isotonicity is the presence of a solution that produces no change in cell volume.