Dispelling Myths and Misconceptions Through the Visualization of










- Slides: 18

Dispelling Myths and Misconceptions Through the Visualization of Quantum Concepts in General Chemistry Morton Hoffman, 1 Dan Dill, 1 Peter Garik, 2 Alex Golger 1 1) Department of Chemistry 2) School of Education Boston University Boston, Massachusetts 02215 http: //quantumconcepts. bu. edu

QC in some current general chemistry textbooks http: //quantumconcepts. bu. edu

What topics are presented? http: //quantumconcepts. bu. edu

What attitudes toward QC do students bring into general chemistry? Fear and loathing from pre-college science courses and the popular culture. l Rumors that it’s about some strange equations and dead, Germanic guys with umlauts in their names. l Concerns that it’s about “mechanics” and other non-inspiring subjects from physics. l http: //quantumconcepts. bu. edu

Do students “get it? ” l l l Most are puzzled about the whole thing. Many see it merely as impenetrable mathematics with no relevance to reality. Some can do the algorithmic “plug-and-chug” calculations without too much difficulty. A few have a satisfactory conceptual understanding. A couple are inspired by it and want to learn more. http: //quantumconcepts. bu. edu

Why should we bother with Quantum Concepts? l l It is basis for understanding spectroscopy, electronic structure, periodic properties. It is the essence of nanotechnology, quantum computing. . . the future. It provides insight into the deeply microscopic (atomic, molecular) world. It encompasses all of chemistry and is completely interdisciplinary. http: //quantumconcepts. bu. edu

General chemistry students and Quantum Concepts l l Quantum Concepts are among the most challenging and unsatisfying topics for students (and instructors). The quantum world makes no sense to everyday intuition; at best, it’s all mathematics. Failure to reconcile this intuition with quantum behavior results in deeply seated myths and misconceptions. Quantum Concepts do not seem to provide useful insights for the rest of general chemistry. http: //quantumconcepts. bu. edu

An e-mail from a chemistry professor at a four-year college I certainly find that chapter (Chapter 6, in Brown and Le. May I think) the hardest to teach. . . because, of course, it's such a skimmed-over thing, without the required mathematics. And then we just “pull out of the hat” things like orbitals and quantum numbers. Once we get into trends and hybridization, things settle down again, but I certainly sense general revolt for a week or two. http: //quantumconcepts. bu. edu

What are some of the prevalent myths and misconceptions about Quantum Concepts? The electron “waves” as it moves. l Through the absorption or emission of light energy, electrons “jump” from one quantum level to another. l Electrons “go around” the atom in a particular quantum state. l Spectral lines represent “energy levels” of the electron. http: //quantumconcepts. bu. edu l

More myths and misconceptions When a “photon” is absorbed, light vanishes; when a “photon” is emitted, light appears. l The “orbital” pictures represent the regions in space in which the electrons move. l The “wavefunction” is a static mathematical representation of the electron in the atom. l http: //quantumconcepts. bu. edu

These myths and misconceptions arise because time has been left out! The Resolution: Include Time! and avoid missed connections http: //quantumconcepts. bu. edu

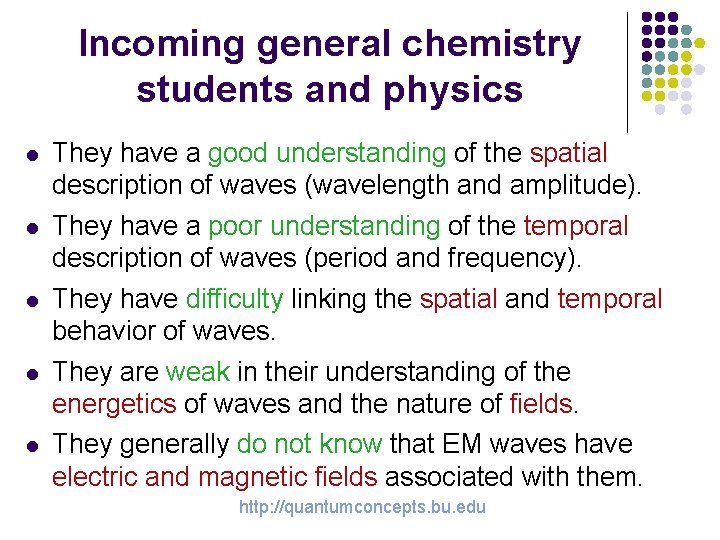
Incoming general chemistry students and physics l They have a good understanding of the spatial description of waves (wavelength and amplitude). l They have a poor understanding of the temporal description of waves (period and frequency). They have difficulty linking the spatial and temporal behavior of waves. They are weak in their understanding of the energetics of waves and the nature of fields. l l l They generally do not know that EM waves have electric and magnetic fields associated with them. http: //quantumconcepts. bu. edu

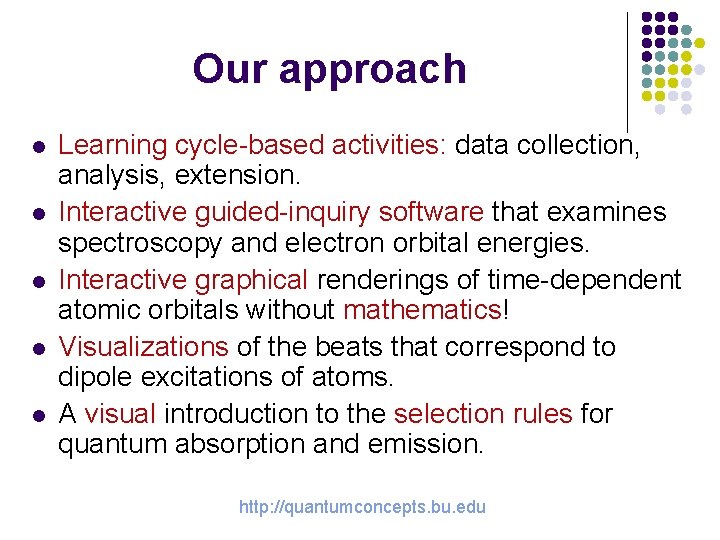
Our approach l l l Learning cycle-based activities: data collection, analysis, extension. Interactive guided-inquiry software that examines spectroscopy and electron orbital energies. Interactive graphical renderings of time-dependent atomic orbitals without mathematics! Visualizations of the beats that correspond to dipole excitations of atoms. A visual introduction to the selection rules for quantum absorption and emission. http: //quantumconcepts. bu. edu

Guided inquiry software Used in conjunction with lecture demonstrations, lecture/discussion workshops, lab exercises, and homework. l l l Project 1: spectroscopy of atomic hydrogen and hydrogen-like ions. Project 2: introduction to the normal modes of one(cable) and two-dimensional (square and circular membranes) waves with analogy to the modes of a bound electron. Project 3: time-dependent behavior of electron orbitals and their interaction with light. http: //quantumconcepts. bu. edu

The Resolution: Include Time! When time is properly included, three key concepts emerge: l l l The electron wavefunction does change with time. Electron density in a specific energy state is nevertheless static: nothing moves, nothing evolves, nothing changes. The mixing of energy states accounts for all motion, evolution, and change. http: //quantumconcepts. bu. edu

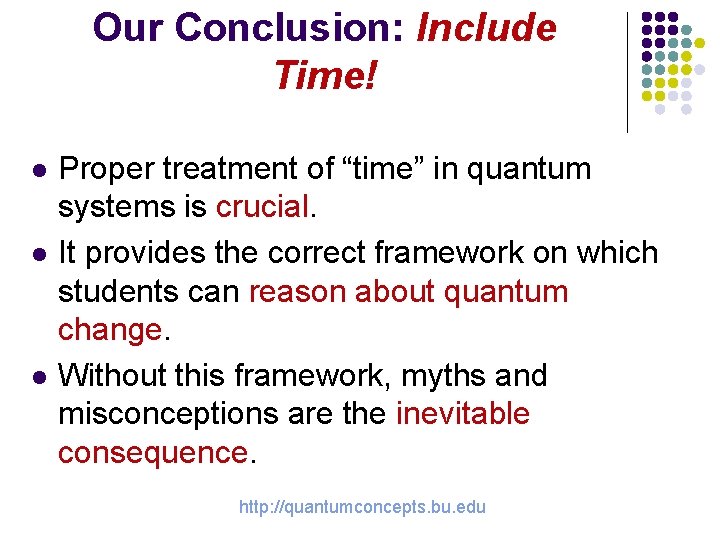
Our Conclusion: Include Time! l l l Proper treatment of “time” in quantum systems is crucial. It provides the correct framework on which students can reason about quantum change. Without this framework, myths and misconceptions are the inevitable consequence. http: //quantumconcepts. bu. edu

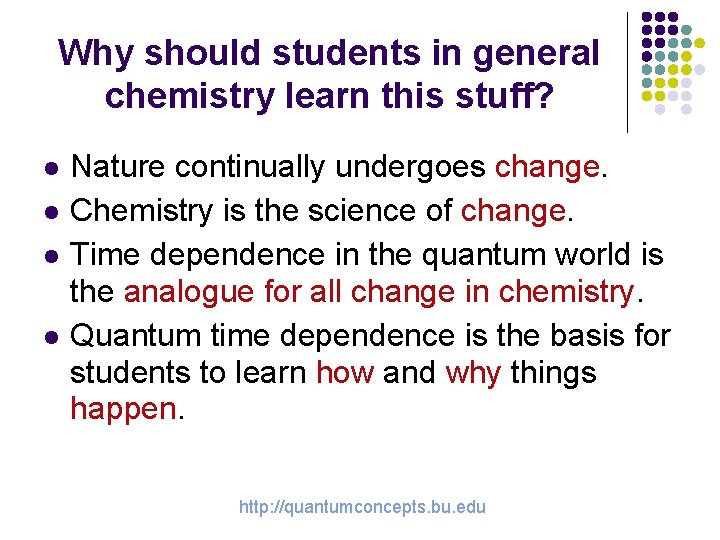
Why should students in general chemistry learn this stuff? l l Nature continually undergoes change. Chemistry is the science of change. Time dependence in the quantum world is the analogue for all change in chemistry. Quantum time dependence is the basis for students to learn how and why things happen. http: //quantumconcepts. bu. edu

Acknowledgements l Peter Carr, Programmer l Joshua Csehak and Lars Travers, Ace Coders Programming l Judith Kelley, Project Evaluator l Funding, U. S. Department of Education Fund for the Improvement of Post Secondary Education (FIPSE Grant P 116 B 020856) http: //quantumconcepts. bu. edu