Disorders of Melanocytes Benign lesionsMelanoma Dr Vishal Mago

Disorders of Melanocytes: Benign lesions/Melanoma Dr Vishal Mago Additional Professor and HOD Department of Burn and Plastic Surgery

Benign-acquired melanocytic nevus • Junctional nevi are small flat lesions that first appear after birth and are smooth, nonpalpable, and light to dark brown or black • nevus is called compound because the central portion is intradermal and thick, whereas the periphery is still junctional and flat. • The intradermal nevus is the common adult mole of the face or trunk • Blue-When the nevus contains melanin that is located more deeply, blue wavelengths of light pass through the less pigmented epidermis and are reflected back to the eye as a blue nevus • Patients with large congenital nevi , greater than 40 m in diameter, were associated with the highest risk of developing melanoma, is present as a pigmented lesion on the day of birth. It may be hairy
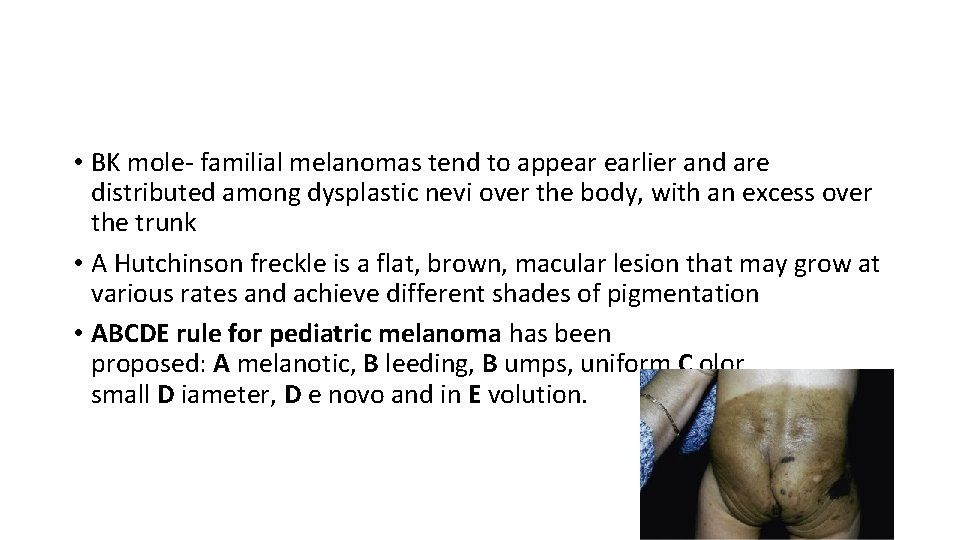
• BK mole- familial melanomas tend to appear earlier and are distributed among dysplastic nevi over the body, with an excess over the trunk • A Hutchinson freckle is a flat, brown, macular lesion that may grow at various rates and achieve different shades of pigmentation • ABCDE rule for pediatric melanoma has been proposed: A melanotic, B leeding, B umps, uniform C olor, small D iameter, D e novo and in E volution.

Risk factors • Risk factors are divided into three major groups: genetic, environmental, and phenotypic • Genetic • Family history of cutaneous melanoma • Lightly pigmented skin • Tendency to burn, inability to tan • Red hair color • DNA repair defects (e. g. xeroderma pigmentosum)
Introduction/Melanoma • A malignant tumor of melanocytes, most commonly arising from cutaneous melanocytes; • Can also develop from melanocytes residing elsewhere – e. g. in the uveal tract, retinal pigment epithelium, gastrointestinal mucosa, or leptomeninges • The UK incidence is 21. 1 per 100 000 per year • The female to male ratio is 2 : 1. • the prognosis is slightly better in females

Environmental • Intense intermittent sun exposure-UVB • Chronic sun exposure • Residence in equatorial latitudes • PUVA (possible) • Tanning bed use, especially under the age of 35 years • Iatrogenic or acquired immunosuppression
Phenotypic • Melanocytic nevi and solar lentigines: • – Increased total number of acquired melanocytic nevi (MN) >100 MN, relative risk ~8 - to 10 -fold increased • – Atypical melanocytic nevi (AMN) >5 AMN, relative risk ~4 - to 6 -fold increased • – Multiple solar lentigines (SL) • Multiple SL, relative risk ~3 - to 4 -fold increased • Relative risks (RR) are multiplicative: e. g. a person with >100 MN + >5 AMN + multiple SL has a relative risk ~ 10 × 5 × 3 = 150 -fold • Personal history of cutaneous melanoma
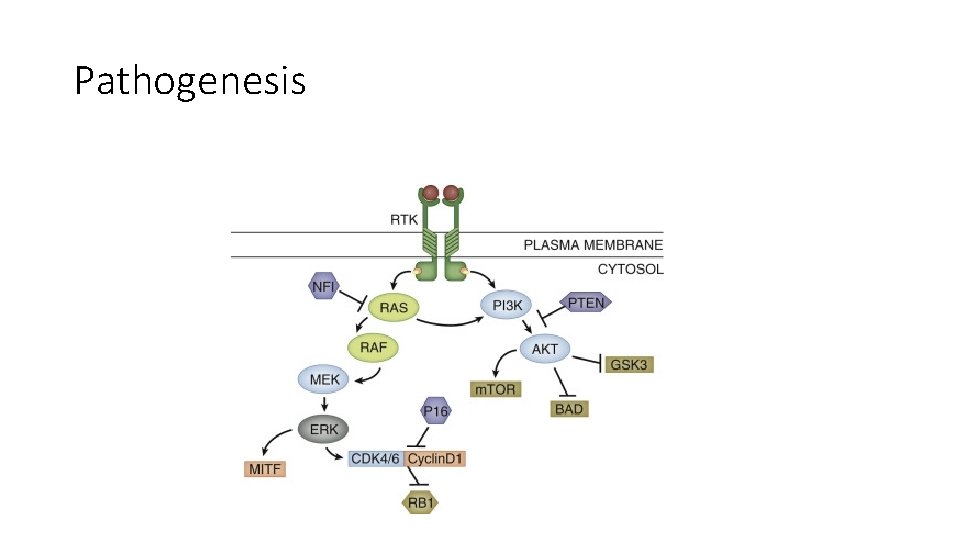
Pathogenesis
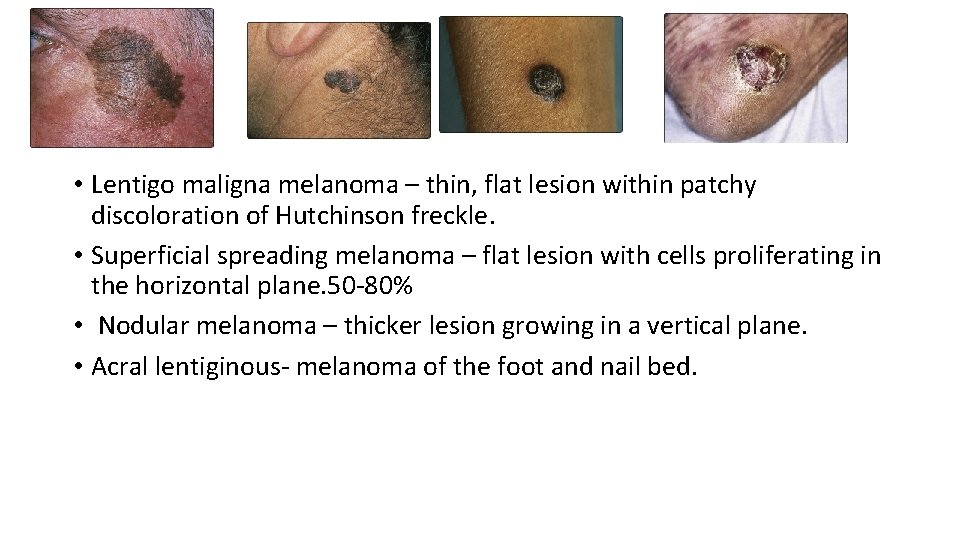
• Lentigo maligna melanoma – thin, flat lesion within patchy discoloration of Hutchinson freckle. • Superficial spreading melanoma – flat lesion with cells proliferating in the horizontal plane. 50 -80% • Nodular melanoma – thicker lesion growing in a vertical plane. • Acral lentiginous- melanoma of the foot and nail bed.
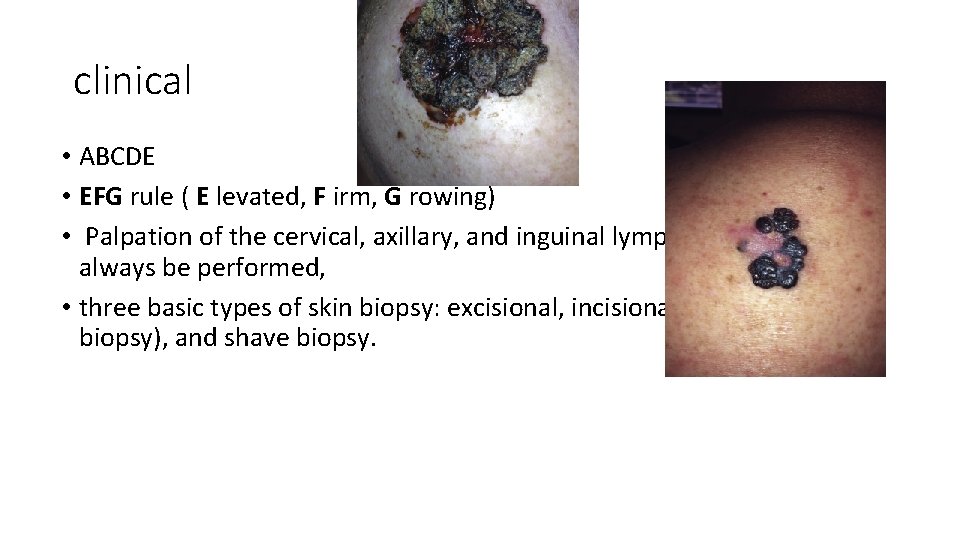
clinical • ABCDE • EFG rule ( E levated, F irm, G rowing) • Palpation of the cervical, axillary, and inguinal lymph nodes should always be performed, • three basic types of skin biopsy: excisional, incisional (including punch biopsy), and shave biopsy.

ABCDEFG mnemonic • A symmetry • B order irregularity • C olor variegation • D iameter more than 6 mm • E levation above skin surface • F eeling different (including pruritus); “F” also is a reminder to check family history • G rowth or change

Diagnosis • Early detection is key to improved survival • diagnosis is based on clinical suspicion, followed by histologic confirmation. • biopsy should attempt to remove the entire lesion, including a depth adequate to determine an accurate Breslow depth • signs such as the ‘ugly duckling sign’ (a pigmented lesion that stands out as atypical within the context of surrounding nevi; and the ‘little red riding hood sign’ can prove useful • Photography and dermoscopy

American Cancer Society as the ABCD Guidelines • Asymmetry of the lesion as it grows from a round or oval lesion • Border irregularity, • Color changes • Diameter of the lesion • the “ugly duckling sign” (i. e. , unique appearance relative to the patient's other nevi), • E for “evolution”, in order to emphasize the significance of evolving pigmented lesions in the natural history
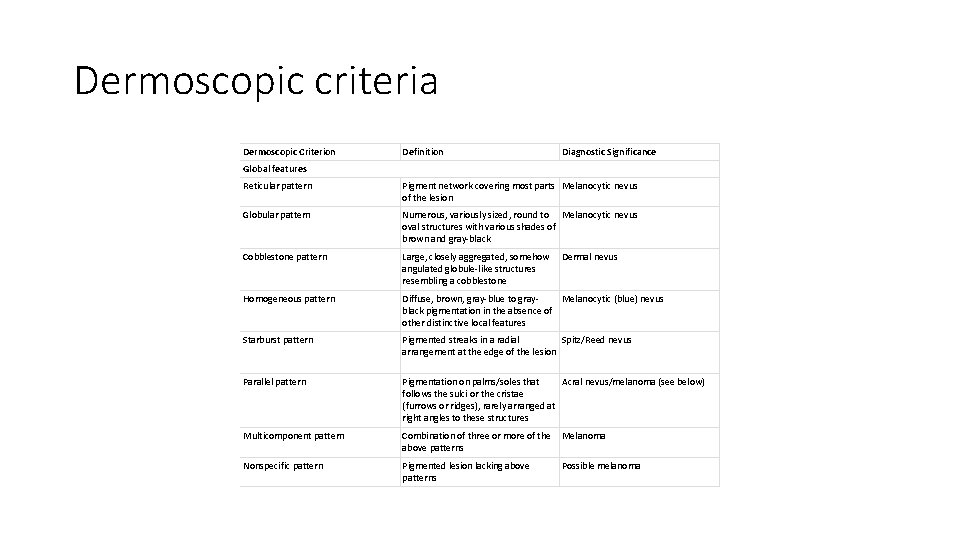
Dermoscopic criteria Dermoscopic Criterion Definition Diagnostic Significance Global features Reticular pattern Pigment network covering most parts Melanocytic nevus of the lesion Globular pattern Numerous, variously sized, round to Melanocytic nevus oval structures with various shades of brown and gray-black Cobblestone pattern Large, closely aggregated, somehow angulated globule-like structures resembling a cobblestone Homogeneous pattern Diffuse, brown, gray-blue to gray. Melanocytic (blue) nevus black pigmentation in the absence of other distinctive local features Starburst pattern Pigmented streaks in a radial Spitz/Reed nevus arrangement at the edge of the lesion Parallel pattern Pigmentation on palms/soles that Acral nevus/melanoma (see below) follows the sulci or the cristae (furrows or ridges), rarely arranged at right angles to these structures Multicomponent pattern Combination of three or more of the above patterns Melanoma Nonspecific pattern Pigmented lesion lacking above patterns Possible melanoma Dermal nevus

• first step is to differentiate melanocytic from nonmelanocytic pigmented lesions by using specific dermoscopic criteria. • second step is to differentiate between the various nonmelanocytic lesions by using other specific dermoscopic criteria
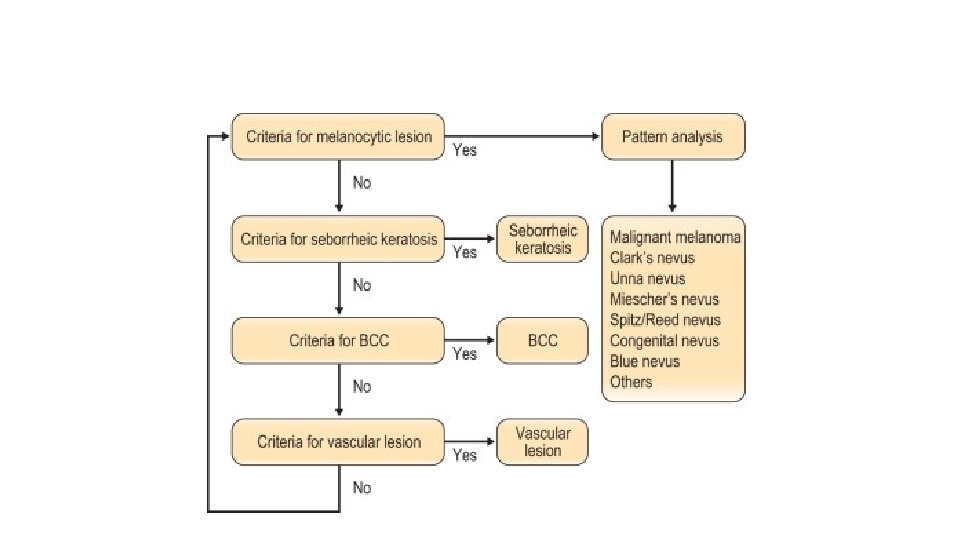
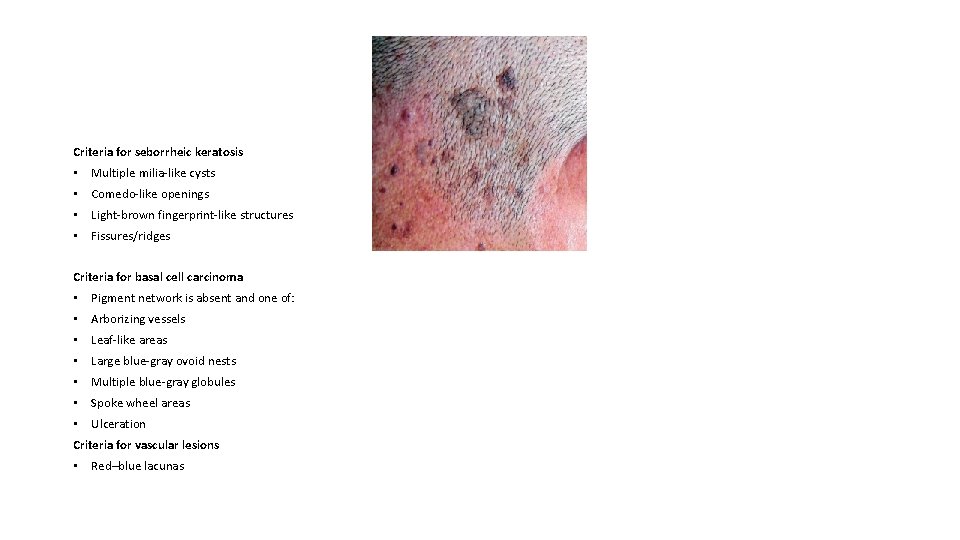
Criteria for seborrheic keratosis • Multiple milia-like cysts • Comedo-like openings • Light-brown fingerprint-like structures • Fissures/ridges Criteria for basal cell carcinoma • Pigment network is absent and one of: • Arborizing vessels • Leaf-like areas • Large blue-gray ovoid nests • Multiple blue-gray globules • Spoke wheel areas • Ulceration Criteria for vascular lesions • Red–blue lacunas

Breslows depth

Clarks levels • Level I: In situ melanoma; limited to the dermis–epidermis junction • Level II: Invading the papillary dermis but without expansion of this layer • Level III: Invading and expanding into the papillary dermis but not into the reticular dermis (to the interface of the papillary–reticular dermis) • Level IV: Invading the reticular dermis, but not into the subcutaneous fat • Level V: Invading the subcutaneous fat or the associated subreticular tissues
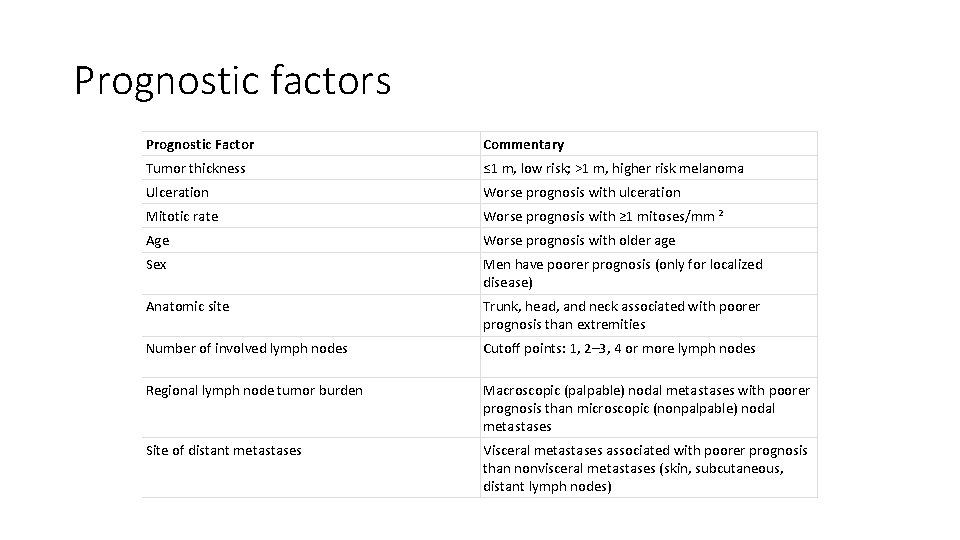
Prognostic factors Prognostic Factor Commentary Tumor thickness ≤ 1 m, low risk; >1 m, higher risk melanoma Ulceration Worse prognosis with ulceration Mitotic rate Worse prognosis with ≥ 1 mitoses/mm 2 Age Worse prognosis with older age Sex Men have poorer prognosis (only for localized disease) Anatomic site Trunk, head, and neck associated with poorer prognosis than extremities Number of involved lymph nodes Cutoff points: 1, 2– 3, 4 or more lymph nodes Regional lymph node tumor burden Macroscopic (palpable) nodal metastases with poorer prognosis than microscopic (nonpalpable) nodal metastases Site of distant metastases Visceral metastases associated with poorer prognosis than nonvisceral metastases (skin, subcutaneous, distant lymph nodes)
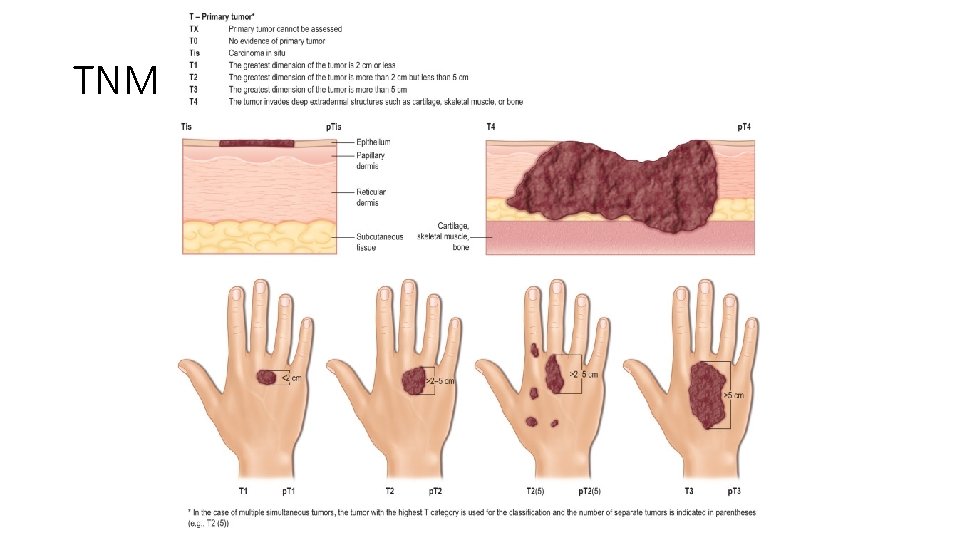
TNM classification
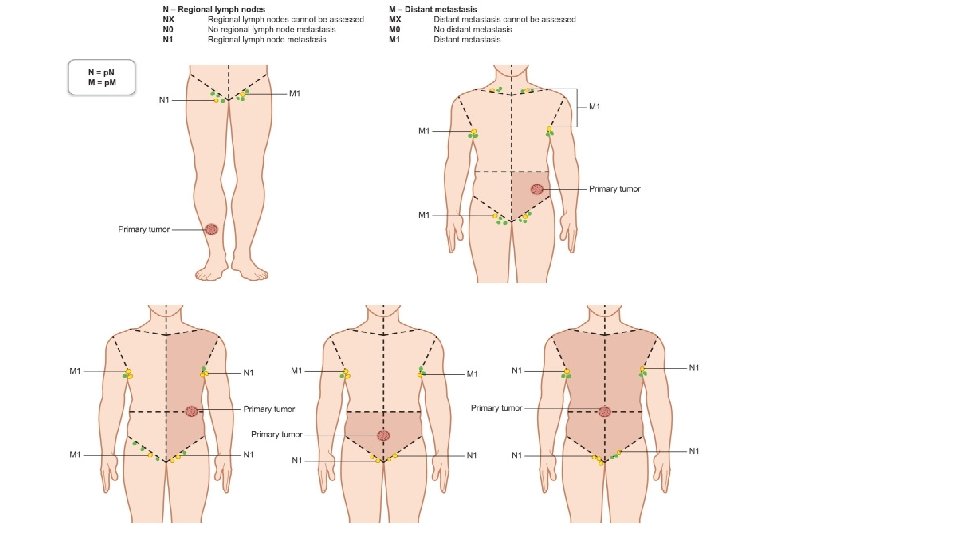

• Nx: Nodes are not assessable (e. g. , previously resected for other reasons) • N 0: No regional lymphatic metastases. • N 1: One involved lymph node. • N 1 a: clinically occult micrometastasis; • N 1 b: clinically detected macrometastasis • N 1 c: in transit or satellite metastasis without metastatic nodes • N 2: Two to three involved nodes • N 2 a: clinically occult micrometastases • N 2 b: at least one node with clinically detected macrometastasis • N 2 c: in-transit or satellite metastasis with one metastatic lymph node • N 3: Four or more positive nodes, matted nodes, or in-transit/satellite metastases with one or more positive nodes • N 3 a: clinically occult micrometastases • N 3 b: one clinically detected micrometastasis • N 3 c: two or more clinically detected micrometastases
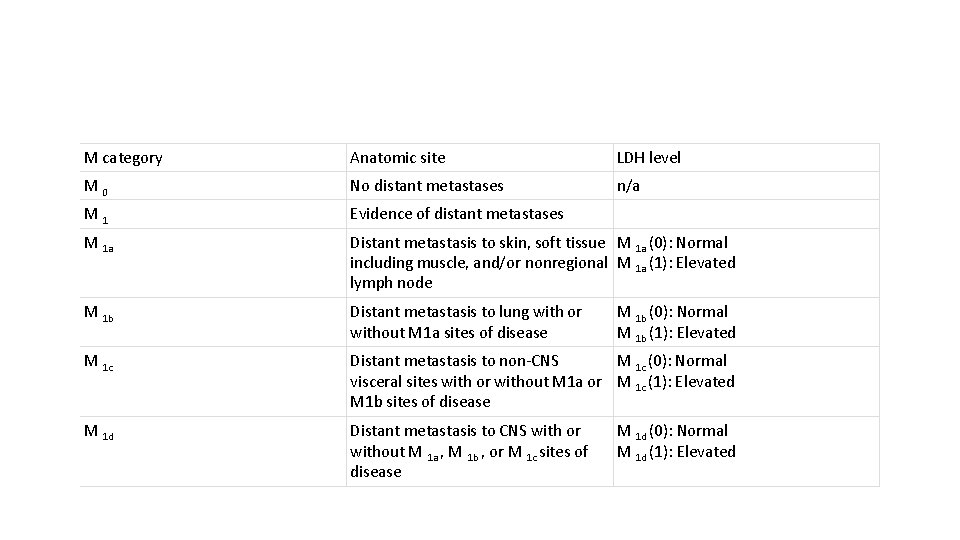
M category Anatomic site LDH level M 0 No distant metastases n/a M 1 Evidence of distant metastases M 1 a Distant metastasis to skin, soft tissue M 1 a (0): Normal including muscle, and/or nonregional M 1 a (1): Elevated lymph node M 1 b Distant metastasis to lung with or without M 1 a sites of disease M 1 c Distant metastasis to non-CNS M 1 c (0): Normal visceral sites with or without M 1 a or M 1 c (1): Elevated M 1 b sites of disease M 1 d Distant metastasis to CNS with or without M 1 a , M 1 b , or M 1 c sites of disease M 1 b (0): Normal M 1 b (1): Elevated M 1 d (0): Normal M 1 d (1): Elevated

Imaging studies Primary tumor (no clinical evidence of other involvement) Physical examination Chest radiography Liver function tests Lymphoscintigraphy to detect sites of sentinel nodes (if primary tumor is 1 m or more thick) Local and regional disease (in-transit lesions or nodal involvement) Physical examination Liver function tests CT scans of chest and abdomen (to examine lungs and liver) of pelvis if tumor involves lower extremities of neck if tumor involves the head and neck Lymphoscintigraphy to detect sites of sentinel nodes Additional scans as indicated by clinical signs or symptoms Distant organ metastases Physical examination Liver function tests and serum lactate dehydrogenase level CT scans as indicated above MRI scans if required to detect extent of soft tissue invasion PET scans to detect extent of tumor involvement of vital organs (lung, liver, brain)
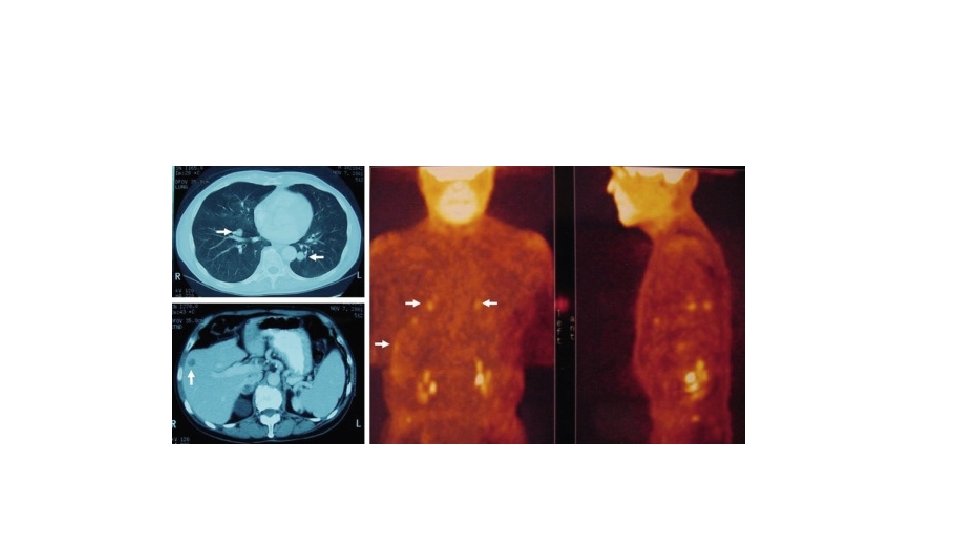

SLNB • SLNB is useful as a diagnostic and prognostic tool • – Indications for consideration of SLNB: CM with T ≥ 1 m and no clinically involved regional lymph nodes or distant metastases; selected patients with T <1 m but with ulceration and/or mitoses ≥ 1/mm 2. • – Typically performed at the time of re-excision • if the sentinel lymph nodes are not involved, the entire basin free of tumor, Breslow depthin 96% of cases. Risk of + SLN <1. 0 m 4– 7% 1. 01– 2. 0 m 12– 20% 2. 01– 4. 0 m 28– 33% >4. 0 m 40– 44%
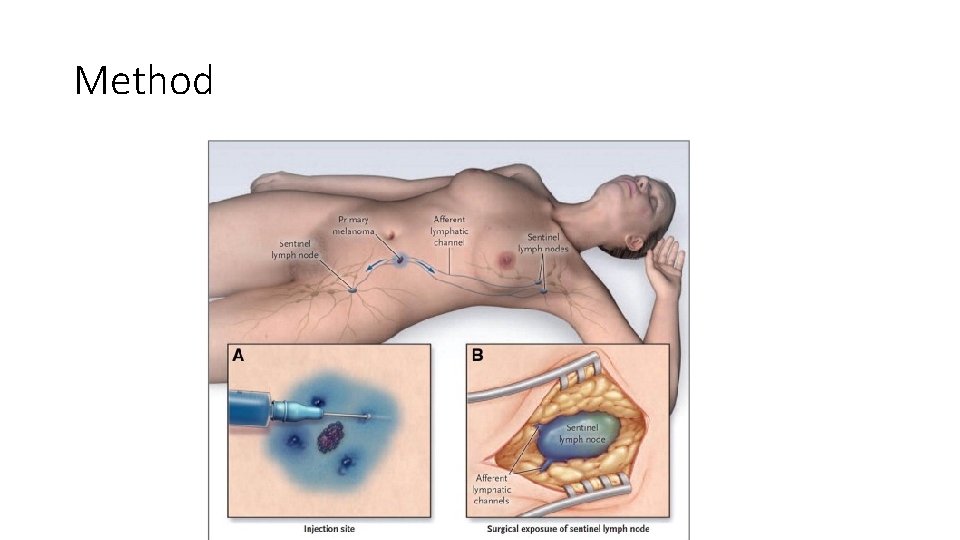
Method

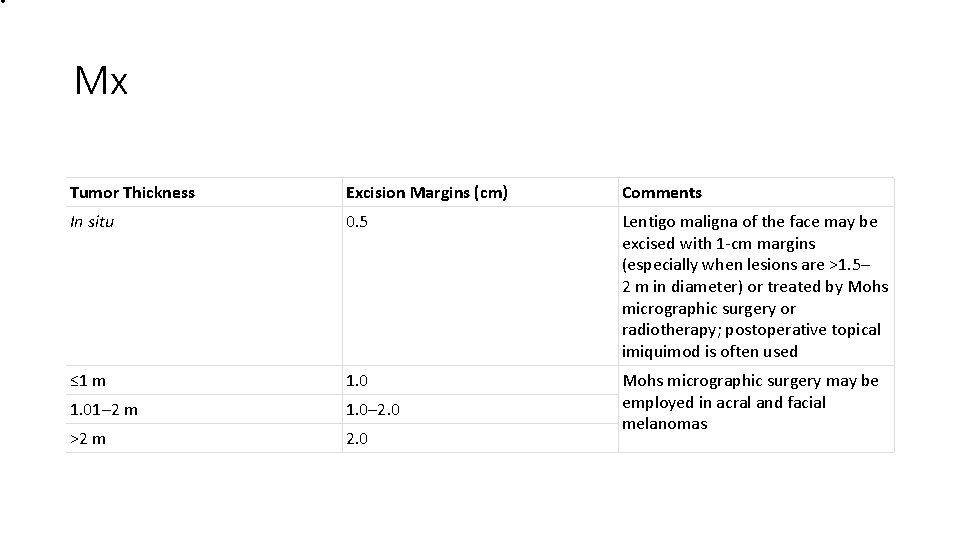
• Mx Tumor Thickness Excision Margins (cm) Comments In situ 0. 5 Lentigo maligna of the face may be excised with 1 -cm margins (especially when lesions are >1. 5– 2 m in diameter) or treated by Mohs micrographic surgery or radiotherapy; postoperative topical imiquimod is often used ≤ 1 m 1. 01– 2 m 1. 0– 2. 0 >2 m 2. 0 Mohs micrographic surgery may be employed in acral and facial melanomas

Management Stage 0 (Melanoma In Situ) • Re-excision Stages I and II • T <1 m (no ulceration and mitoses <1/mm 2 ) • – Re-excision • T ≥ 1 m or T <1 m but with ulceration and/or mitoses ≥ 1/mm • – Consider SLNB before performing re-excision 2 Stage III • Lymph node metastases, including micrometastases noted by SLNB • – Consider clinical trial enrollment or interferon- α • In-transit metastases • – 1 st: <5, surgery vs. >5, extremity perfusion * • – 2 nd: Consider topical therapy (e. g. imiquimod, miltefosine) • – 3 rd: Systemic therapy Stage IV • Skin or subcutaneous metastases • – Single to a few: Surgery • – Multiple: Surgery vs. topical (see above) vs. systemic therapy • Solid organ metastases • – Systemic therapy +/− surgery or radiotherapy (including stereotactic)

LND • Offer therapeutic lymph node dissection to people with palpable stage IIIB–IIIC melanoma or nodal disease detected by imaging. • If the SLN is positive, the patient should be offered complete dissection of the involved lymph node basin • Patients with melanoma of the face and anterior scalp who are selected for cervical lymphadenectomy - positive sentinel lymph nodes should also be considered for superficial parotidectomy • the use of carbon to pinpoint the intranodal site of metastases and the application of molecular staging will decide ELND

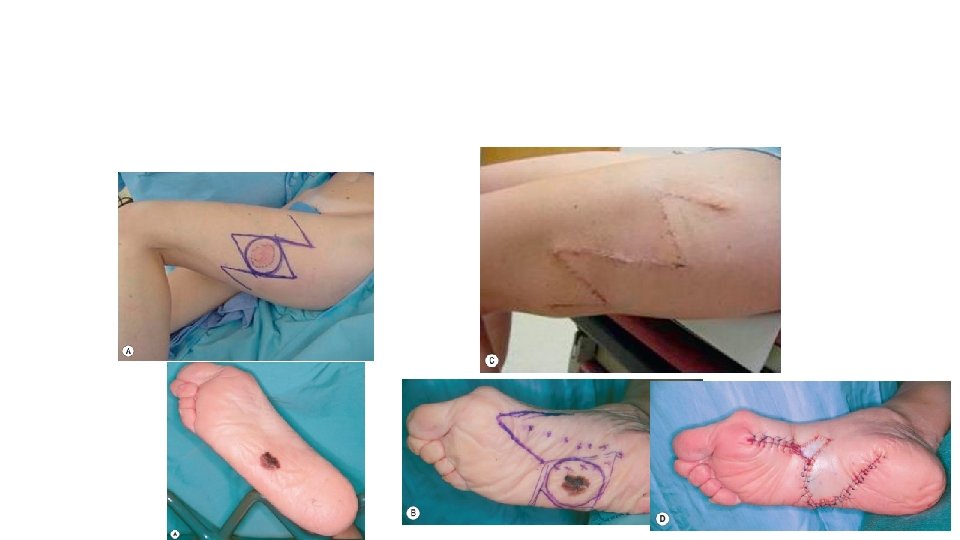
Wide local excision is the standard of care for early stage melanoma, and the majority of patients present with stage I–IIA disease

Immunotherapy Mechanism Examples CTLA-4 inhibition • Ipilimumab (IV) PD-1 inhibition • Nivolumab (IV) • Lambrolizumab (IV) PD-L 1 inhibition • RG-7446, MEDI-4736 Mutated Oncogene-Directed Therapy Mutated Oncogene Examples c-KIT • Imatinib • Dasatinib NRAS • Tipifarnib BRAF • Selective for V 600 E * • Vemurafenib • Dabrafenib • Nonselective • Sorafenib MEK 1/2 • Trametinib

Surveillance Office visits and physical examination Stage 0 Yearly by dermatologist or primary care physician Stage I Every 6 months for 5 years Stage II–IV Every 3 months for 2 years Every 6 months for 3 years Metastatic surveillance Lactate dehydrogenase At each visit, at least yearly level and complete blood cell count Chest radiograph Every other visit, at least yearly CT scan ± PET With laboratory test or chest radiograph abnormality, physical findings, or symptoms
- Slides: 36